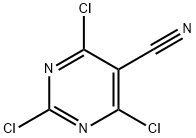
2,4,6-TRICHLORO-5-CYANOPYRIMIDINE synthesis
- Product Name:2,4,6-TRICHLORO-5-CYANOPYRIMIDINE
- CAS Number:3029-64-9
- Molecular formula:C5Cl3N3
- Molecular Weight:208.43
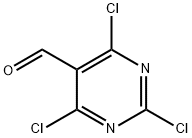
50270-27-4
256 suppliers
$23.00/1g

3029-64-9
118 suppliers
inquiry
Yield: 92%
Reaction Conditions:
Stage #1:2,4,6-trichloropyrimidine-5-carbaldehyde with hydroxylamine hydrochloride;acetic acid in water at 60; for 1 h;
Stage #2: with thionyl chloride at 20; for 2.16667 h;Reflux;
Steps:
2.2 Step 2. 2,4,6-trichioropyrimidin-5-formonitrile
Step 2.
2,4,6-trichloropyrimidin-5-formonitrile
2,4,6-trichloropyrimidin-5-carboxaldehyde (10 g, 47.4 mmol), hydroxylamine hydrochloride (3.3 g, 47.5 mmol), glacial acetic acid (100 mL), and H2O (5 mL) were added to a 150-mL round-bottom flask, and heated to 60° C. to react for 1 hour.
After the completion of the reaction, the reaction solution was poured into crushed ice, and was extracted with CH2Cl2.
The resultant organic phases were combined, washed with a saturated NaCl solution, dried with anhydrous Na2SO4, and then filtered and concentrated.
The obtained substance was dissolved in SOCl2 (60 mL), reacted at room temperature for 10 minutes, and then heated for a reflux reaction for 2 hours.
After the completion of the reaction, the resultant mixture was subjected to reduced pressure distillation to remove SOCl2 to give a residue, which was then dissolved in EtOAc and washed with H2O.
The resultant organic phase was dried with anhydrous Na2SO4, filtered, and concentrated to obtain an oily substance of 9 g.
The yield was 92%.
References:
Shenzhen Bo Li Jian Medicine Co., Ltd.;Ye, Baohuan US2019/255057, 2019, A1 Location in patent:Paragraph 0086; 0088
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

3029-64-9
118 suppliers
inquiry