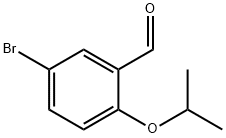
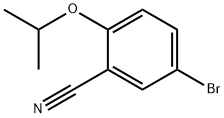
5-BROMO-2-ISOPROPOXYBENZALDEHYDE synthesis
- Product Name:5-BROMO-2-ISOPROPOXYBENZALDEHYDE
- CAS Number:138505-25-6
- Molecular formula:C10H11BrO2
- Molecular Weight:243.1
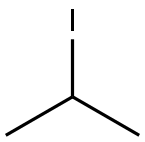
75-30-9
263 suppliers
$15.00/1g
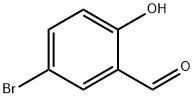
1761-61-1
379 suppliers
$5.00/5g

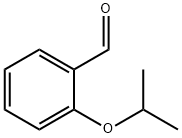
138505-25-6
48 suppliers
$58.00/250mg
Yield:138505-25-6 99%
Reaction Conditions:
with potassium carbonate;caesium carbonate in N-methyl-acetamide;
Steps:
29.A Synthesis of DArPhin Catalysts
Step A: Synthesis of 5-bromo-2-isopropoxybenzaldehyde AX. To a suspension of potassium carbonate (34.4 g, 249 mmol) and cesium carbonate (16.2 g, 50 mmol) in dimethylformamide were added 5-bromosalicaldehyde (25.0 g, 124 mmol) and 2-iodopropane (25.0 mL, 249 mmol). The suspension was stirred at room temperature overnight, then at 70° C. for 4 hrs. The volatiles were removed, and the residue was partitioned between methyl t-butylether and water. The aqueous layer was extracted with methyl t-butylether and the combined organic phases were washed with water, sodium hydroxide, and brine, and then dried over magnesium sulfate. Concentration to dryness afforded compound AX (30.0 g) as a pale yellow oil in 99% yield. 1H NMR (CDCl3, 400 MHz): δ 1.40 (d, J=6.3 Hz, 6H), 4.65 (sept., J=6.0 Hz, 1H), 6.89 (d, J=9.0 Hz, 1H), 7.59 (dd, J=9.0 and 2.7 Hz, 1H), 7.91 (d, J=2.7 Hz, 1H), 10.39 (s, 1H).
References:
US2009/202480,2009,A1
22921-58-0
75 suppliers
$42.00/500mg

138505-25-6
48 suppliers
$58.00/250mg
515832-52-7
46 suppliers
$43.29/250mg

138505-25-6
48 suppliers
$58.00/250mg
75-26-3
456 suppliers
$10.00/10g

1761-61-1
379 suppliers
$5.00/5g

138505-25-6
48 suppliers
$58.00/250mg
40530-18-5
179 suppliers
$15.00/1g

138505-25-6
48 suppliers
$58.00/250mg