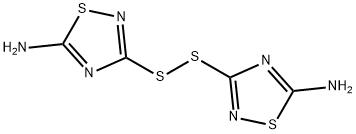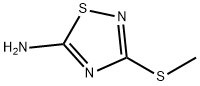
5-AMINO-3-METHYLTHIO-1,2,4-THIADIAZOLE synthesis
- Product Name:5-AMINO-3-METHYLTHIO-1,2,4-THIADIAZOLE
- CAS Number:6913-13-9
- Molecular formula:C3H5N3S2
- Molecular Weight:147.22

540-72-7
444 suppliers
$13.50/100G
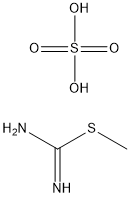
867-44-7
366 suppliers
$5.00/10g

6913-13-9
34 suppliers
$45.00/10mg
Yield:6913-13-9 69.3%
Reaction Conditions:
Stage #1: sodium thiocyanide;S-Methylisothiourea sulfate in methanol at -5;
Stage #2: with bromine;triethylamine in methanol at 5 - 25; for 2 h;
Steps:
1 Reference Example 1
Reference Example 1 (0026) (0027) 900 mL (milliliters; hereinafter, the same) of methanol was stirred, and 230 mL (1.1 mol) of a 28 mass % methanol solution of sodium methoxide was added thereto. Subsequently, 139 g (0.5 mol) of compound A was added thereto. The internal temperature was adjusted to -5° C. using dry ice-methanol, and then 163 g (1.02 mol) of bromine and 205 mL (1.0 mol) of a 28 mass % methanol solution of sodium methoxide were simultaneously added dropwise thereto at an internal temperature of 5° C. or lower. After completion of the dropwise addition, the mixture was stirred for 2 hours at an internal temperature of 25° C., and then an inorganic salt thus precipitated was collected by filtration. To the filtrate, an aqueous solution obtained by dissolving 6.4 g of sodium hydrogen sulfite in 35 mL of water was added. Subsequently, the mixture was distilled off under reduced pressure, and a concentrated residue was extracted with water. Thus, 51 g of compound B was obtained (yield: 69.3%).
References:
US2017/81517,2017,A1 Location in patent:Paragraph 0026; 0027

540-72-7
444 suppliers
$13.50/100G

62-56-6
529 suppliers
$18.00/50g

6913-13-9
34 suppliers
$45.00/10mg

540-72-7
444 suppliers
$13.50/100G
2986-19-8
67 suppliers
inquiry

6913-13-9
34 suppliers
$45.00/10mg

867-44-7
366 suppliers
$5.00/10g
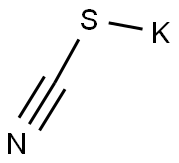
333-20-0
452 suppliers
$13.50/100G

6913-13-9
34 suppliers
$45.00/10mg