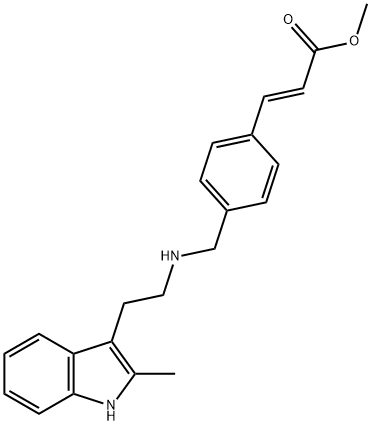
2-Propenoic acid, 3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]aMino]Methyl]phenyl]-, Methyl ester, (2E)- synthesis
- Product Name:2-Propenoic acid, 3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]aMino]Methyl]phenyl]-, Methyl ester, (2E)-
- CAS Number:441741-65-7
- Molecular formula:C22H24N2O2
- Molecular Weight:348.44
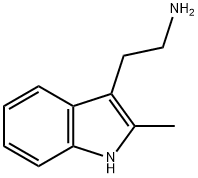
2731-06-8
139 suppliers
$50.00/1g
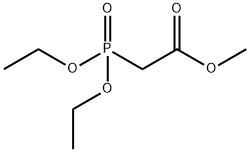
1067-74-9
217 suppliers
$5.00/5g

73291-09-5
113 suppliers
$65.00/100mg
![2-Propenoic acid, 3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]aMino]Methyl]phenyl]-, Methyl ester, (2E)-](/CAS/GIF/441741-65-7.gif)
441741-65-7
15 suppliers
inquiry
Yield:441741-65-7 55%
Reaction Conditions:
Stage #1: 2-methyltryptamine;Methyl diethylphosphonoacetatewith 1,8-diazabicyclo[5.4.0]undec-7-ene;lithium chloride in N,N-dimethyl-formamide at 0; for 0.25 h;Inert atmosphere;
Stage #2: 4-chloromethylbenzaldehyde in N,N-dimethyl-formamide at 0; for 6 h;Inert atmosphere;
Steps:
2; 3; 4; 5 Example 4(E)-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]Methyl acrylate Preparation
2-methyltryptamine (1.2 g, 6.7 mmol), DMF (10 ml), DBU (3.8 g, 25 mmol), lithium chloride (0.04 g, 1 mmol),Methyl phosphonoacetate diethyl ester (4.2 g, 20 mmol) in a 50 ml single-mouth bottle,N2 protection, stirring at 0 ° C for 15 min,4-Chloromethylbenzaldehyde (1.5 g, 10 mmol) in DMF (5 ml) was added dropwiseSolution, same temperature reaction6h,Add 50ml of water,Extract with ethyl acetate (50 ml * 3), combine the organic phases, respectively, 50 ml with water,Wash with saturated sodium chloride solution (50 ml * 2), dry over anhydrous sodium sulfate and suction.The solvent was dried and dissolved in ethyl acetate.Add 1,4-dioxane/HCl solution to the acid,A white solid of 1.65 g was obtained by suction filtration, yield 55%.
References:
CN108752255,2018,A Location in patent:Paragraph 0030-0032; 0035-0043
100-63-0
362 suppliers
$10.00/1g
![2-Propenoic acid, 3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]aMino]Methyl]phenyl]-, Methyl ester, (2E)-](/CAS/GIF/441741-65-7.gif)
441741-65-7
15 suppliers
inquiry
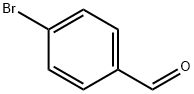
1122-91-4
686 suppliers
$9.00/10g
![2-Propenoic acid, 3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]aMino]Methyl]phenyl]-, Methyl ester, (2E)-](/CAS/GIF/441741-65-7.gif)
441741-65-7
15 suppliers
inquiry
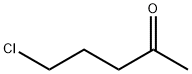
5891-21-4
361 suppliers
$5.00/5g
![2-Propenoic acid, 3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]aMino]Methyl]phenyl]-, Methyl ester, (2E)-](/CAS/GIF/441741-65-7.gif)
441741-65-7
15 suppliers
inquiry

2731-06-8
139 suppliers
$50.00/1g
![2-Propenoic acid, 3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]aMino]Methyl]phenyl]-, Methyl ester, (2E)-](/CAS/GIF/441741-65-7.gif)
441741-65-7
15 suppliers
inquiry