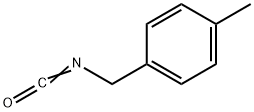
4-Methylbenzyl isocyanate synthesis
- Product Name:4-Methylbenzyl isocyanate
- CAS Number:56651-57-1
- Molecular formula:C9H9NO
- Molecular Weight:147.17
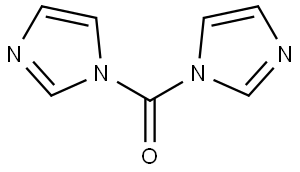
530-62-1
935 suppliers
$6.00/25g
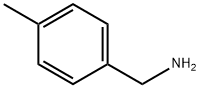
104-84-7
219 suppliers
$21.00/5g

56651-57-1
68 suppliers
$60.00/100mg
Yield: 100%
Reaction Conditions:
in tetrahydrofuran at 70;Product distribution / selectivity;
Steps:
79
To a solution of 4-methylbenzylamine (1.5 mL, 1 1.8 mmol) in THF (30mL) added CDI (2.1 mol eq, 3.8g) and the mixture was heated at 70°C overnight. The reaction mixture was evaporated, water was added and the aqueous phase was extracted with EtOAc (3x30 mL). The recombined organic phases were anhydrified over Na2SO4 and evaporated at reduced pressure to give a white solid (1 1.78 mmol, 2.53g, quantitative yield). The solid obtained (0.83 g, 3.88 mmol) was dissolved in DMF (20 mL) and the bicyclic amine If was added (0.8 mol eq, 0.62g), then the mixture obtained was heated at 100°C overnight. The solvent was removed at reduced pressure and the residue was purified by crystallization from hot MeOH to give the product as pale yellow solid (0.078g, 8%Yield). 1HNMR (DMSO, 200 MHz) δ 2.27 (s, 3H), 4.24 (d, 2H, J=6), 4.59 (s, 2H), 6.28 (t, 1H), 4.47 (dd, 1H), 6.80 (t, 1H, J=8), 7.1 1-7.21 (m, 4H), 7.77 (dd, 1H, J=2), 8.07 (s, 1H), 10.63 (bs, 1H). [M+1] 31 1.99 (C17H17N3O3 requires 31 1.34).
References:
PHARMESTE S.R.L.;NAPOLETANO, Mauro;TREVISANI, Marcello;PAVANI, Maria Giovanna;FRUTTAROLO, Francesca WO2011/120604, 2011, A1 Location in patent:Page/Page column 116; 117
32315-10-9
416 suppliers
$10.00/1g

104-84-7
219 suppliers
$21.00/5g

56651-57-1
68 suppliers
$60.00/100mg
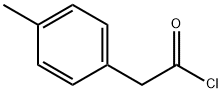
35675-44-6
36 suppliers
$38.00/250mg

56651-57-1
68 suppliers
$60.00/100mg
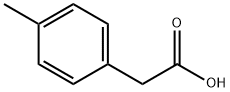
622-47-9
400 suppliers
$17.00/25g

56651-57-1
68 suppliers
$60.00/100mg
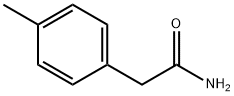
6212-86-8
8 suppliers
$33.39/250mg

56651-57-1
68 suppliers
$60.00/100mg