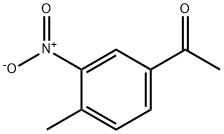
4-Methyl-3-nitroacetophenone synthesis
- Product Name:4-Methyl-3-nitroacetophenone
- CAS Number:5333-27-7
- Molecular formula:C9H9NO3
- Molecular Weight:179.17
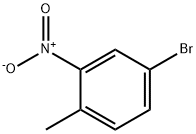
60956-26-5
289 suppliers
$8.00/10g
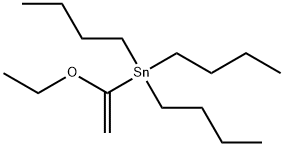
97674-02-7
229 suppliers
$15.00/1 g

5333-27-7
142 suppliers
$43.20/5g
Yield: 82.7%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane at 100; for 12 h;Inert atmosphere;
Steps:
1.1 Step 1: l-(4-Methyl-3-nitro-phenyl)ethanone
To a solution of 4-bromo-l-methyl-2-nitro-benzene (2.0 g, 9.26 mmol,1 eq) in 1,4- dioxane (20 mL) was added tributyl(l -ethoxy vinyl)stannane (3.84 g,10.63 mmol, 3.59 mL,1.15 eq) and Pd(dppf)Ch (338.70 mg, 462.89 pmol, 0.05 eq) under N2. The mixture was stirred at 100 °C for 12 h. The reaction mixture was cooled to 0°C and treated with 20 ml 2N hydrochloric acid and stirred for 1 h. Last the reaction mixture was adjusted pH to 8 with aq. NaOH (15%). TLC (PE/EtOAc = 5/1, Rf = 0.27) indicated the starting material was consumed completely and two new spots formed. The mixture was added aq. KF (80 mL) stirred for 1 h. The mixture was extracted with EtOAc (50 mL x 3) and the combined organic layers were washed with brine (50 mL), dried over Na2S04, filtered and concentrated to yield a residue which was purified by flash silica gel chromatography (From PE/EtOAc = 1/0 to 5/1, Rf = 0.30) to yield l-(4-methyl-3-nitro- phenyl)ethanone (1.43 g, 7.66 mmol, 82.7% yield, 96.0% purity) as a yellow solid. NMR (400 MHz, DMSO- e) d ppm 8.42 (s,1H), 8.15 (d, J= 8.2 Hz,1H), 7.66 (d, J= 7.8 Hz,1H), 2.62 (s, 3H), 2.57 (s, 3H);
References:
IKENA ONCOLOGY, INC. WO2021/127301, 2021, A1 Location in patent:Paragraph 00221; 00363
122-00-9
693 suppliers
$5.00/25g

5333-27-7
142 suppliers
$43.20/5g
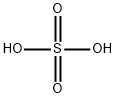
7664-93-9
6 suppliers
$21.64/1003
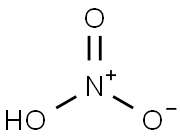
7697-37-2
8 suppliers
$13.00/25g

122-00-9
693 suppliers
$5.00/25g

5333-27-7
142 suppliers
$43.20/5g