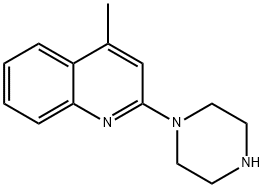
4-METHYL-2-PIPERAZIN-1-YL-QUINOLINE synthesis
- Product Name:4-METHYL-2-PIPERAZIN-1-YL-QUINOLINE
- CAS Number:50693-78-2
- Molecular formula:C14H17N3
- Molecular Weight:227.3
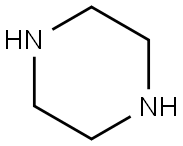
110-85-0
564 suppliers
$5.00/5G
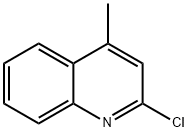
634-47-9
167 suppliers
$42.00/5g

50693-78-2
28 suppliers
$110.00/50mg
Yield:50693-78-2 86%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 140;
Steps:
11.1 Step 1. Formation of 4-methyl-2-(piperazin-1-yl)quinoline
0478] To a solution of 2-chloro-4-methylquinoline (2 g, 11 mmol) in N,N-dimethylformamide (40 ml) was added piperazine (4.86 g, 56.4 mmol, 5 eq) and potassium carbonate (2.34 g, 16.8 mmol, 1.5 eq). The mixture was stirred overnight at 140° C., quenched by the addition of water (200 ml), and extracted with ethyl acetate (3×100 ml). The organic layers were combined and washed with saturated aqueous sodium chloride (200 ml). The ethyl acetate solution was dried over anhydrous sodium sulfate, filtered, and concentrated under vacuum. The crude material was purified by silica gel chromatography using 1-10% methanol in dichloromethane to elute. The product-containing fractions were combined and concentrated to afford 4-methyl-2-(piperazin-1-yl)quinoline as colorless oil (2.2 g, 86%); (ES, m/z) [M+H]+ 228; 1H NMR (300 MHz, CDCl3): δ 7.70-7.78 (m, 2H), 7.50-7.55 (t, J=7.5 Hz, 1H), 7.22-7.27 (t, J=6.9 Hz, 1H), 6.83 (s, 2H), 3.70-3.73 (t, J=4.8 Hz, 1H), 3.00-3.03 (t, J=5.1 Hz, 1H), 2.59 (s, 3H).
References:
US2014/142114,2014,A1 Location in patent:Paragraph 0477; 0478
62-53-3
658 suppliers
$10.00/1g

50693-78-2
28 suppliers
$110.00/50mg
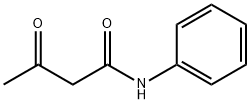
102-01-2
8 suppliers
$5.00/100g

50693-78-2
28 suppliers
$110.00/50mg
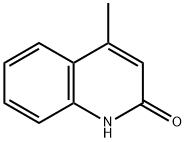
607-66-9
186 suppliers
$8.00/5g

50693-78-2
28 suppliers
$110.00/50mg