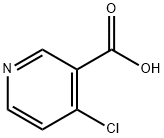
4-Chloronicotinic acid synthesis
- Product Name:4-Chloronicotinic acid
- CAS Number:10177-29-4
- Molecular formula:C6H4ClNO2
- Molecular Weight:157.55
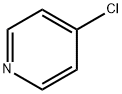
626-61-9
50 suppliers
$365.00/500mg
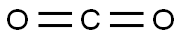
124-38-9
130 suppliers
$175.00/23402

10177-29-4
303 suppliers
$6.00/1g
Yield:10177-29-4 80%
Reaction Conditions:
Stage #1:4-Chloropyridine with lithium diisopropyl amide in tetrahydrofuran;hexane at -78;Inert atmosphere;
Stage #2:carbon dioxide in tetrahydrofuran;hexane
Steps:
2
As above, 4-chloropyridine 1 is obtained by neutralization of 4-chloropyridine hydrochloride with 10% NaOH as described in SCHMID & WOLKOFF (Canadian Journal of Chemistry, vol. 50, p. 1181-1187, 1972). 4-Chloropyridine 1 (15 mmol) is reacted in THF (250 ml) at -78° C. (nitrogen atmosphere) with 1.2 equivalents of lithium diisopropylamide (1.5 M solution in hexanes containing one equivalent of THF, ALDRICH) (THRASHER et al., Heterocycles, vol. 67, p. 543-547, 2006).Reaction of the resulting anion with dry CO2 allows the formation of 4-chloropyridine-3-carboxylic acid 3 (4-chloronicotinic acid), isolated as a colorless solid with a yield of 60-80% (see GUILLIER et al., J. Org. Chem., vol. 60, p. 292-296, 1995).
References:
CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (CNRS;INSTITUT CURIE;UNIVERSITE MONTPELLIER 2 SCIENCES ET TECHNIQUES US2011/53975, 2011, A1 Location in patent:Page/Page column 60
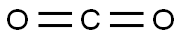
124-38-9
130 suppliers
$175.00/23402

10177-29-4
303 suppliers
$6.00/1g
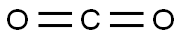
124-38-9
130 suppliers
$175.00/23402
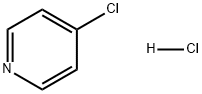
7379-35-3
426 suppliers
$5.00/1g

10177-29-4
303 suppliers
$6.00/1g
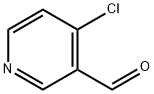
114077-82-6
161 suppliers
$11.00/250mg

10177-29-4
303 suppliers
$6.00/1g
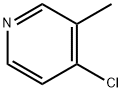
1681-36-3
111 suppliers
$70.00/500 mg

10177-29-4
303 suppliers
$6.00/1g