
4-Chlorobenzenamine hydrochloride synthesis
- Product Name:4-Chlorobenzenamine hydrochloride
- CAS Number:20265-96-7
- Molecular formula:C6H7Cl2N
- Molecular Weight:164.03
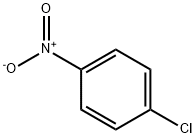
100-00-5
10 suppliers
$14.00/25g

20265-96-7
204 suppliers
$28.67/5g
Yield:20265-96-7 100%
Reaction Conditions:
Stage #1: 4-chlorobenzonitrilewith 1% platinum on charcoal;ammonia;hydrogen;N-Cyanoguanidine in toluene at 90 - 100; under 7500.75 Torr; for 6 h;Autoclave;Large scale;
Stage #2: with hydrogenchloride in water at 90 - 100;Large scale;Reagent/catalyst;Solvent;Temperature;Pressure;Time;Concentration;
Steps:
1; 2; 3 Example 3 Preparation of p-chloroaniline hydrochloride
Into a 2000L autoclave, 800L of toluene, 400kg of p-chloronitrobenzene, 1.2kg of 1% platinum-carbon wet product (0.4kg on dry basis, 66.7% of wet product moisture content), 40g of dicyandiamide, and 1.6kg of ammonia were sealed. The autoclave was evacuated for 10 minutes. Nitrogen was used to replace the air three times, and hydrogen was continuously substituted to replace the nitrogen three times. The pressure of the hydrogen gas was controlled to 1.0 MPa. The reaction solution was heated to 90-100 ° C and hydrogenated for 6 hours until the reaction solution ceased. Absorb hydrogen, reduce the temperature of the reaction solution to below 60 ° C, replace the hydrogen with nitrogen, filter off the catalyst by nitrogen pressure (to be applied), collect the filtrate into a 100L enamel reactor, add 4kg of activated carbon to decolorize, and add 424kg of concentrated hydrochloric acid to the filtrate. Warm to 90 ° C for 1 hour, continue to raise the temperature to 100 ° C, reflux through a water trap, toluene with water until no more water is separated, a large amount of white solids are suspended, cool the reaction solution to room temperature, and centrifuge to remove the mother liquor. A small amount of toluene was used to rinse the filter cake, and the filter cake was dried under reduced pressure at 80 ° C for 4 hours to obtain 400 kg of white crystal p-chloroaniline hydrochloride, with a weight yield of 100%, a molar yield of 96%, a purity of 99.94%, and a content of 99.89%.
References:
CN110467533,2019,A Location in patent:Paragraph 0109-0114; 0119; 0124; 0132; 0140-0141; 0145; 0149
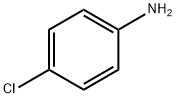
106-47-8
474 suppliers
$5.00/5G

20265-96-7
204 suppliers
$28.67/5g

100-00-5
10 suppliers
$14.00/25g

20265-96-7
204 suppliers
$28.67/5g
142-04-1
193 suppliers
$10.00/10g
539-03-7
310 suppliers
$10.00/5G

20265-96-7
204 suppliers
$28.67/5g
3289-75-6
95 suppliers
$24.00/1g

20265-96-7
204 suppliers
$28.67/5g