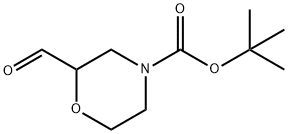
4-BOC-2-MORPHOLINECARBALDEHYDE synthesis
- Product Name:4-BOC-2-MORPHOLINECARBALDEHYDE
- CAS Number:218594-02-6
- Molecular formula:C10H17NO4
- Molecular Weight:215.25
135065-69-9
145 suppliers
$8.00/250mg

218594-02-6
58 suppliers
$124.00/100mg
Yield: 67%
Reaction Conditions:
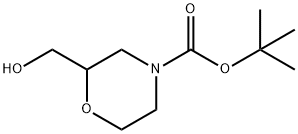
Stage #1:tert-butyl 2-(hydroxymethyl)morpholine-4-carboxylate with oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -78; for 1.16667 h;
Stage #2: with triethylamine in dichloromethane for 0.5 h;
Steps:
1 Step 1.
To a stirred solution of oxylyl chloride (1.93 g, 15.2 mmol) in DCM (40 mL) was slowly added DMSO (0.79 mL, 11.0 mmol) at -78 °C, and the resulting mixture was stirred for 30 min at this temperature. A solution of tert-butyl 2-(hydroxymethyl)morpholine-4-carboxylate (3.00 g, 13.8 mmol) in DCM (10 mL) was added slowly over 10 min and stirred for 1 h. Then TEA (6.29 g, 62.1 mmol) was added dropwise and stirred for 0.5 h. The reaction mixture was allowed to warm slowly to rt, and then quenched with water (30 mL). After extraction with DCM (20 mL × 3), the organic layer was washed successively with HCl (10 mL, 1 M), saturated aqueous Na2CO3 (30 mL), and then brine (30 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo to afford tert-butyl 2-formylmorpholine-4-carboxylate as a yellow oil (2 g, 67% yield). LCMS: LC retention time 1.49 min; MS (ESI) m/z 160 [M-t-Bu]+.
References:
GENZYME CORPORATION WO2021/97057, 2021, A1 Location in patent:Page/Page column 445