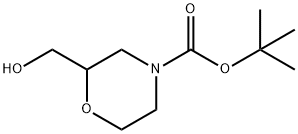
2-HYDROXYMETHYL-4-BOC-MORPHOLINE synthesis
- Product Name:2-HYDROXYMETHYL-4-BOC-MORPHOLINE
- CAS Number:135065-69-9
- Molecular formula:C10H19NO4
- Molecular Weight:217.26
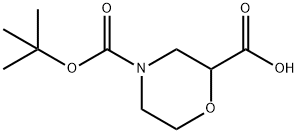
189321-66-2
145 suppliers
$5.00/250mg

135065-69-9
145 suppliers
$8.00/250mg
Yield:135065-69-9 97%
Reaction Conditions:
Stage #1:4-(tert-butoxycarbonyl)morpholine-2-carboxylic acid with borane-THF in tetrahydrofuran at 0 - 20; for 0.416667 h;
Stage #2: with methanol;acetic acid in tetrahydrofuran
Steps:
2.a
A solution of 4-{[(1 ,1-dimethylethyl)oxy]carbonyl}-2-morpholinecarboxylic acid (2.0 g, 21.6 mmol) in THF (45 mL) was cooled to 0 °C. A solution of borane (39 mL, 39.0 mmol, 1 M in THF) was added over 25 min via addition funnel. After warming to RT, the reaction was quenched by dropwise addition of methanol/acetic acid (18 mL, 9:1 v/v). The solvent was removed under reduced pressure and the residue partitioned between ethyl acetate and 1 N HCI. The aqueous layer was extracted with ethyl acetate and combined extracts were washed with water, 1 N NaOH, water, brine and dried over sodium sulfate. Removal of the solvent under reduced pressure afforded 1.83 g (97%) of the desired material. 1H NMR (400 MHz, CDCI3) δ ppm 1.49 (s, 9 H) 2.30 (br d, J=11.37 Hz, 1 H) 2.69 - 2.79 (m, J=9.51 , 6.41 , 3.28, 3.28 Hz, 1 H) 2.84 (ddd, J=13.77, 10.86, 3.16 Hz, 2 H) 3.27 - 3.38 (m, 1 H) 3.47 (br s, 1 H) 3.63 - 3.75 (m, 2 H) 4.10 - 4.19 (m, 1 H) 4.27 (br s, 1 H).
References:
SMITHKLINE BEECHAM CORPORATION WO2007/58850, 2007, A2 Location in patent:Page/Page column 43

24424-99-5
833 suppliers
$13.50/25G
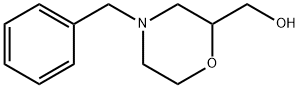
40987-24-4
110 suppliers
$32.00/250mg

135065-69-9
145 suppliers
$8.00/250mg

24424-99-5
833 suppliers
$13.50/25G
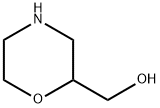
103003-01-6
113 suppliers
$45.00/50mg

135065-69-9
145 suppliers
$8.00/250mg
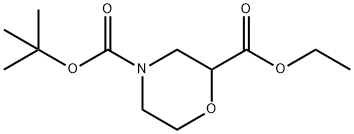
768371-16-0
70 suppliers
$18.00/250mg

135065-69-9
145 suppliers
$8.00/250mg
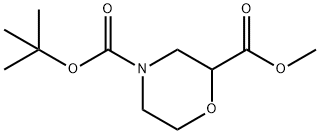
500789-41-3
64 suppliers
$21.00/100mg

135065-69-9
145 suppliers
$8.00/250mg