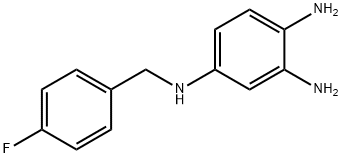
4-(4-Fluorobenzylamino)-1,2-phenylenediamine synthesis
- Product Name:4-(4-Fluorobenzylamino)-1,2-phenylenediamine
- CAS Number:491871-67-1
- Molecular formula:C13H14FN3
- Molecular Weight:231.27
![2-Amino-5-[(4-fluorobenzyl)amino]-1-nitrobenzene](/CAS/GIF/150812-21-8.gif)
150812-21-8
119 suppliers
$37.70/1g

491871-67-1
19 suppliers
inquiry
Yield:491871-67-1 99.6%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in tetrahydrofuran
Steps:
4.1 4.1. Synthesis of tert-butyl 2-(tert-butoxycarbonyl imino)-4-(N-parafluorobenzyl-imino)-aniline formate
Preparation example4: synthesis of ethyl 2-amino-4-(N-parafluorobenzyl-N-allyl-imino) -aniline formate (K20) RRN 54,594.1. Synthesis of tert-butyl 2-(tert-butoxycarbonyl imino)-4-(N-parafluorobenzyl-imino)-aniline formate
According to a procudure similar to that of Preparation example 1, intermediate 2-nitro -4-(N-parafluorobenzyl-imino)-aniline was obtained by using o-nitro paraphenylene diamine and parafluorobenzaldehyde as starting materials. 2-nitro-4-(N-parafluorobenzyl-imino)-aniline (2.61g, 0.01mol) was dissolved in THF (30mL) and air therein was replace with N2.
Then Pd-C (10%, 261mg) was rapidly added and N2 therein was replaced with H2.
The hydrogenation reaction was conducted overnight, and the resulting resction solution was filtered and concentrated to produce a product of 2-amino-4-(N-parafluorobenzyl-imino)-aniline (2.3g, 99.6%, colorless oil, instable, prone to be oxidized and deteriorated), which was rapidly used in nest step. 1H NMR(300 MHz, CDCl3): δ 7.32(t, J=8.7Hz, 2H), 7.02(t, J=8.7Hz, 2H), 6.58(d, J=8.7Hz, 1H), 6.01-6.08(m, 2H), 4.22(s, 2H).
References:
Shanghai Institute Materia Medica, Chinese Academy Of Sciences;NAN, Fajun;LI, Min;GAO, Zhaobing;CHEN, Fei;ZHANG, Yangming;ZHOU, Pingzheng;HU, Haining;XU, Haiyan;LIU, Sheng EP2772481, 2014, A1 Location in patent:Paragraph 0051-0052
![2-AMino-4-[(4-fluorobenzyl)aMino]-1-nitrobenzene(RETIGABINE inteMediate)](/CAS/GIF/1263404-74-5.gif)
1263404-74-5
27 suppliers
inquiry

491871-67-1
19 suppliers
inquiry
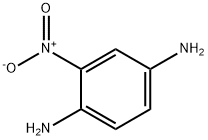
5307-14-2
346 suppliers
$5.00/10g

491871-67-1
19 suppliers
inquiry
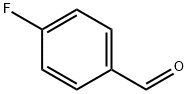
459-57-4
594 suppliers
$6.00/25g

491871-67-1
19 suppliers
inquiry
![(1E)-1-N-[(4-Fluorophenyl)methylidene]-3-nitrobenzene-1,4-diamine](/CAS/GIF/150812-22-9.gif)
150812-22-9
5 suppliers
inquiry

491871-67-1
19 suppliers
inquiry