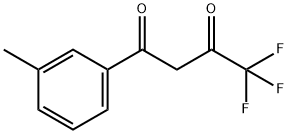
4,4,4-TRIFLUORO-1-(3-METHYLPHENYL)-1,3-BUTANEDIONE synthesis
- Product Name:4,4,4-TRIFLUORO-1-(3-METHYLPHENYL)-1,3-BUTANEDIONE
- CAS Number:53764-99-1
- Molecular formula:C11H9F3O2
- Molecular Weight:230.18
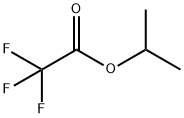
400-38-4
191 suppliers
$21.00/5g
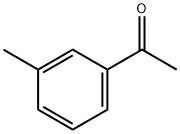
585-74-0
181 suppliers
$10.00/5g

53764-99-1
72 suppliers
$11.00/100mg
Yield:53764-99-1 87%
Reaction Conditions:
with sodium methylate in toluene; for 4 h;Reflux;
Steps:
Synthetic procedure for Impurity A (2)
To a solution of isopropyl 2,2,2-trifluoroacetate 10 (7.40g, 0.0474mol) in toluene (15 mL) was added and 3’-methylacetophenone 11 (6.36g, 0.0474mol) and cooled to 0°C, followed by dropwise addition Sodium methoxide (3.0g, 0.0616 mol) to the reaction mixture. The reaction mixture was heated to reflux. After stirring for 4 h, the reaction mixture was diluted with water (275 mL), brine (275 mL), EtOAc (500 mL). The aqueous layer was separated and extracted with EtOAc (200 mL x 4). The combined organic phases were washed with brine (500 mL x 1), dried over Na2SO4 and concentrated under vacuum. The crude product purified by flash column chromatography to give 4,4,4-trifluoro-1-(m-tolyl)butane-1,3-dione, 8 as a white solid (9.5g, 87% yield). To a 500 mL round-bottomed flask containing ethyl acetate (20 mL) and water (16 mL) was added 4,4,4-trifluoro-1-(m-tolyl)butane-1,3-dione 8 (4.0g, 0.0174mol), the reaction mixture was cooled to 0°C and stirred for 15 min. 4-hydrazinobenzenesulfonamide hydrochloride7 (4.0g, 0.0214 mol) was added to the reaction mixture slowly. The reaction was refluxed for 8 h then cooled to rt. The solid precipitated on cooling was filtered, the filtered solid was washed with cold isopropyl alcohol (20mL X 2) to give the the impurity A 2 as white solid (5.15g) with an excellent yield of 92.0%. 4-(5-(m-tolyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzenesulfonamide (2) 1H NMR (400 MHz, DMSO - d6) δ 7.94 - 7.79 (m, 2H), 7.60 - 7.45 (m, 4H), 7.21 (dd, J = 10.9, 5.1 Hz, 4H), 7.08 - 6.89 (m, 1H), 2.25 (s, 3H). 13C NMR(101 MHz, DMSO - d6) δ 145.82, 144.54, 142.86, 142.45, 141.59, 138.80, 130.57, 130.04, 129.18, 128.69, 127.31, 126.51, 121.86 (q, J = 265 Hz), 106.91, 21.42. 19F NMR (376 MHz, DMSO - d6) δ -60.77. HRMS m/z (M-H)-: 380.0695; calculated for C17H14F3N3O2S; 380.0686
References:
Lee, Young Hee;Vishwanath, Manjunatha;Lanka, Srinu;Lee, Eunhwa;Park, Yongbin;Lee, Sunhwan;Sim, Jaeuk;Lee, Seohoo;Lee, Kiho;Viji, Mayavan;Lee, Heesoon;Jung, Jae-Kyung [Bulletin of the Korean Chemical Society,2019,vol. 40,# 6,p. 479 - 480] Location in patent:supporting information
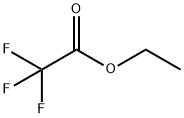
383-63-1
484 suppliers
$10.00/25g

585-74-0
181 suppliers
$10.00/5g

53764-99-1
72 suppliers
$11.00/100mg