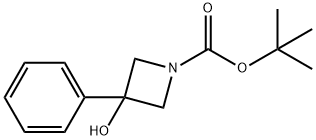
3-HYDROXY-3-PHENYLAZETIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:3-HYDROXY-3-PHENYLAZETIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:398489-25-3
- Molecular formula:C14H19NO3
- Molecular Weight:249.31
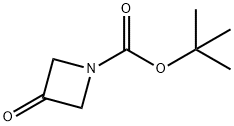
398489-26-4
427 suppliers
$9.00/5g
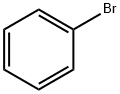
108-86-1
507 suppliers
$10.00/5g

398489-25-3
33 suppliers
$170.00/250MG
Yield:398489-25-3 94%
Reaction Conditions:
Stage #1: bromobenzene in tetrahydrofuran at -78; for 0.166667 h;Inert atmosphere;
Stage #2: with n-butyllithium in tetrahydrofuran;hexane; for 1 h;Inert atmosphere;
Stage #3: tert-butyl 3-oxoazetidine-1-carboxylate in tetrahydrofuran at 20;Inert atmosphere;
Steps:
N-Boc-3-aryl-azetidin-3-ol (4). General Procedure.
General procedure: The corresponding halogenated bromobenzene (3 equiv) was dissolved in anhydrous THF (10mL) and cooled to -78 °C for 10 minutes. A solution of n-butyllithium (2.5M in hexane, 3 equiv) was added dropwise and the reaction mixture was stirred for 1 h under nitrogen. N-Boc-3-azetidinone (1 equiv) was dissolved in anhydrous THF (1 mL), and added via syringe to the reaction mixture. The mixture was stirred overnight and allowed to warm to room temperature.The reaction was quenched with cold 10% NH4Cl (10 mL, 0 °C), extracted with diethyl ether (3x 50 mL). The combined organic portions were washed with brine, dried over anhydrous sodium sulfate and the solvent was removed under reduced pressure. The residue was purified by column chromatography (SiO2, 30% ethyl acetate:hexanes) to afforded the desired N-Boc-3-arylazetidin-3-ol (4).
References:
Thaxton, Amber;Izenwasser, Sari;Wade, Dean;Stevens, Edwin D.;Mobley, David L.;Jaber, Vivian;Lomenzo, Stacey A.;Trudell, Mark L. [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 15,p. 4404 - 4407] Location in patent:supporting information

398489-26-4
427 suppliers
$9.00/5g
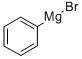
100-58-3
190 suppliers
$18.00/10ml

398489-25-3
33 suppliers
$170.00/250MG

398489-26-4
427 suppliers
$9.00/5g
100-59-4
261 suppliers
$42.00/0.25mole

398489-25-3
33 suppliers
$170.00/250MG