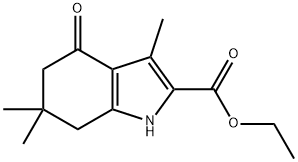
3,6,6-TRIMETHYL-4-OXO-4,5,6,7-TETRAHYDRO-1H-INDOLE-2-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:3,6,6-TRIMETHYL-4-OXO-4,5,6,7-TETRAHYDRO-1H-INDOLE-2-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:37711-24-3
- Molecular formula:C14H19NO3
- Molecular Weight:249.31
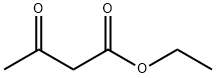
141-97-9
790 suppliers
$5.00/25g
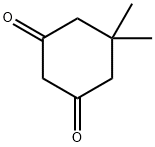
126-81-8
407 suppliers
$5.00/10g

37711-24-3
20 suppliers
$175.00/1g
Yield:-
Reaction Conditions:
Stage #1: ethyl acetoacetatewith acetic acid;sodium nitrite in water at 12 - 20;
Stage #2: dimedonewith zinc in water at 60; for 3.5 h;Reflux;
Steps:
4.i i) Synthesis of ethyl 3,6,6-trimethyl-4-oxo-4,5,6, 7-tetrahydro-1H-indole-2-carboxylate
i) Synthesis of ethyl 3,6,6-trimethyl-4-oxo-4,5,6, 7-tetrahydro-1H-indole-2-carboxylate (1008) [00163] To a solution of ethyl acetoacetate (1 mL) in acetic acid (2.6 mL) was added NaNC>2 (0.67 g) in water (2.2 mL) while maintaining the temperature of the reaction mixture < 12 °C. The resultant solution was stirred at a temperature <12 °C for 3 h and then at room temperature overnight. To this, 5,5-dimethyl-1 ,3-cyclohexanedione (1.51 g) in acetic acid (5.1 mL) was added followed by Zn dust (1.41 g), while maintaining the temperature of the reaction around 60 °C. The mixture was stirred for 30 min, then heated to reflux for 3 h. The solution was separated from Zn dust by filtering through a plug of Celite. The filtrate was poured into ice-cold water and the precipitate was collected by suction filtration, then purified by flash column chromatography (EtOAc/cyclohexane) to obtain the desired product. [00164] 1 H NMR (400 MHz, CDCb) δ 9.54 (1 H, br. s, NH), 4.33 (2H, q, J = 7.1 Hz, CO2CH2CH3), 2.66 (2H, s, CHz), 2.59 (3H, s, CH3), 2.34 (2H, s, CH2), 1.37 (3H, t, J = 7.1 Hz, CO2CH2CH3), 1.09 (6H, s, 2xCH3); HRMS (TOF, ESI") m/z: [M-H]" Calcd for Ci4Hi803N 248.12922; Found 248.12913.
References:
WO2018/215799,2018,A1 Location in patent:Paragraph 00163; 00164

126-81-8
407 suppliers
$5.00/10g
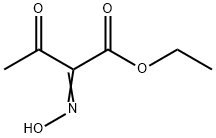
5408-04-8
67 suppliers
$32.00/500mg

37711-24-3
20 suppliers
$175.00/1g