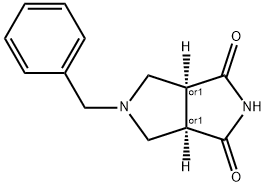
CIS-5-BENZYLTETRAHYDROPYRROLO[3,4-C]PYRROLE-1,3(2H,3AH)-DIONE synthesis
- Product Name:CIS-5-BENZYLTETRAHYDROPYRROLO[3,4-C]PYRROLE-1,3(2H,3AH)-DIONE
- CAS Number:370879-53-1
- Molecular formula:C13H14N2O2
- Molecular Weight:230.26
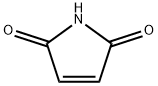
541-59-3
373 suppliers
$8.00/5g
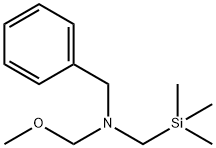
93102-05-7
306 suppliers
$6.00/1g
![CIS-5-BENZYLTETRAHYDROPYRROLO[3,4-C]PYRROLE-1,3(2H,3AH)-DIONE](/CAS/GIF/370879-53-1.gif)
370879-53-1
49 suppliers
$70.00/50mg
Yield: 83%
Reaction Conditions:
Stage #1:maleiimide;N-benzyl-N-(methoxymethyl)-N-[(trimethylsilyl)methyl]amine with trifluoroacetic acid in dichloromethane at 0 - 20;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water
Stage #3: with hydroxylamine in methanol;water at 20; for 20 h;
Steps:
1.1A
Example 1; N-(3,5-dimethylphenyl)-6-((3aR,6aS)-hexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl)pyrazine-2-carboxamide; Example 1A; (3aR,6aS)-5-benzyltetrahydropyrrolo[3,4-c]pyrrole-1,3(2H,3aH)-dione; A solution of N-benzyl-N-methoxymethyl-N-(trimethylsilylmethyl)amine (76.68 g, 0.29 mol, prepared as in Organic Syntheses (1989), 67, 133-140) in dichloromethane (130 mL) was added to an ice-cooled mixture of maleimide (25.53 g, 0.26 mol) and trifluoroacetic acid (2.2 mL, 0.028 mol) in dichloromethane (350 ml) over 40 min so that the reaction temperature remained between 0-5° C. The resulting bright yellow solution was allowed to warm gradually and stirred at room temperature for 27 h. The mixture was washed with saturated NaHCO3(aq) (80 mL) and the organic phase was dried (MgSO4) and concentrated under vacuum. The residual oil was stirred with 10% EtOAc-heptane (300 mL) for 15 h, and the resulting precipitate was isolated by filtration, washed with 10% EtOAc-heptane (150 mL) and dried under vacuum at 50° C. to provide the crude, title compound as a white solid (54.25 g). This was stirred with methanol (500 mL) and 50% aqueous H2NOH (4.2 mL) at room temperature for 20 h. The mixture was concentrated under vacuum and the residue was taken up in EtOAc (500 mL) and filtered to remove some insoluble material. The filtrate was concentrated under vacuum to leave the pure, title compound as an off-white solid (49.9 g, 83% yield). 1H NMR (300 MHz, CD3OD) δ ppm 2.32-2.43 (m, 2H), 3.18 (d, J=9.8 Hz, 2H), 3.20-3.26 (m, 2H), 3.59 (s, 2H), 7.13-7.35 (m, 5H); MS (DCI/NH3) m/z 231 (M+H)+.
References:
Abbott Laboratories US2009/281118, 2009, A1 Location in patent:Page/Page column 10