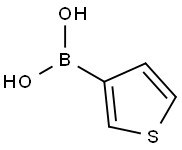
3-Thiopheneboronic acid synthesis
- Product Name:3-Thiopheneboronic acid
- CAS Number:6165-69-1
- Molecular formula:C4H5BO2S
- Molecular Weight:127.96
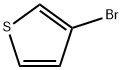
872-31-1
348 suppliers
$9.00/1g
5419-55-6
376 suppliers
$12.00/5g

6165-69-1
341 suppliers
$12.72/1gm:
Yield:6165-69-1 80%
Reaction Conditions:
with hydrogenchloride;n-butyllithium in tetrahydrofuran;hexane
Steps:
150.A A.
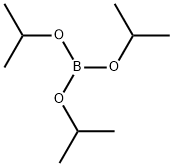
A. 3-Thiopheneboric acid To a solution of 3-bromothiophene (8.15 g, 50 mmol) in THF (20 ml) at -78° C. under an argon atmosphere was added n-butyllithium (2.5M solution in hexane, 20 ml, 50 mmol) dropwise and the resultant solution was stirred at -78° C. for 45 min. This solution was added to a solution of triisopropyl borate (9.4 g, 50 mmol) in THF at -78° C. over 30 min through a steel cannula. The resultant reaction mixture was stirred at room temperature for 12 h and was decomposed by the addition of 100 ml 1N HCl. The aqueous layer was extracted with ether (2*100 ml) and the combined organic layer was extracted with 1M NaOH (3*30 ml), the aqueous extract was acidified with concentrated HCl to pH 2 and extracted with ether (3*50 ml). The combined ether extract was washed once with water, dried over MgSO4 and filtered. Removal of the solvent gave 3-thenylboronic acid as a solid (5.2 g, 80% yield).
References:
Texas Biotechnology Corporation US5571821, 1996, A

872-31-1
348 suppliers
$9.00/1g

6165-69-1
341 suppliers
$12.72/1gm:

872-31-1
348 suppliers
$9.00/1g

5419-55-6
376 suppliers
$12.00/5g

6165-69-1
341 suppliers
$12.72/1gm:

872-31-1
348 suppliers
$9.00/1g
13675-18-8
160 suppliers
$6.00/1g

6165-69-1
341 suppliers
$12.72/1gm:
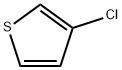
17249-80-8
214 suppliers
$6.00/1g

6165-69-1
341 suppliers
$12.72/1gm: