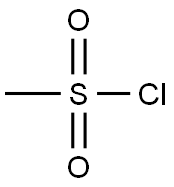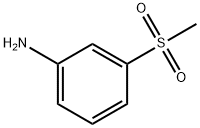
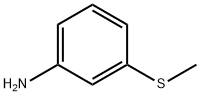
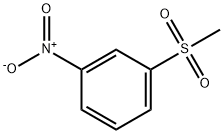
3-(METHYLSULFONYL)ANILINE synthesis
- Product Name:3-(METHYLSULFONYL)ANILINE
- CAS Number:35216-39-8
- Molecular formula:C7H9NO2S
- Molecular Weight:171.22
1783-81-9
203 suppliers
$20.00/25g

35216-39-8
72 suppliers
$19.00/100mg
Yield: 92%
Reaction Conditions:
with sodium tungstate;dihydrogen peroxide;acetic acid in water at 65; for 1.5 h;
Steps:
E Oxidation of methylthio aniline to methylsulfonyl aniline
General procedure: A mixture of Na2W04 (0.067 g), 1 drop of acetic acid, and H20 (5 mL) was placed in a flask and heated to 65 °C. Methylthioaniline 153, 154, or 161 (500 mg, 3.59 mmol) was added, followed by dropwise addition of H202 (1.1 mL, 10.77 mmol). The mixture was stirred at 65 °C for 1.5 h and, after cooling, 80 mL of 1 N HC1 and 50 mL of DCM were added. The layers were separated, and the aqueous phase was washed with additional DCM. The aqueous phase was basified with 25% NaOH and extracted with DCM. The organic phase was washed with brine and dried over Na2S04. The solvent was removed to give methylsulfonyl aniline derivatives 157, 158 and 164. [0232] 3-(methylsulfonyl)aniline (158). Compound 158 was prepared according to procedure E from 153 to afford 557 mg of brown powder (92%). 1H NMR (500 MHz, CDC13) δ 3.03 (s, 3H), 6.89 (ddd, J = 0.8, 2.3, 8.0 Hz, 1H), 7.21 (t, J = 2.1Hz, 1H), 7.24 (dt, J = 1.1, 7.7Hz, 1H), 7.30 (t, 7.8Hz, 1H). 13C NMR (126 MHz, CDC13) δ 44.40, 112.69, 116.37, 119.72, 130.30, 141.16, 147.69. LCQ (M+H+) calcd C7H9N02S 172, found 172.
References:
NORTHWESTERN UNIVERSITY;SILVERMAN, Richard, B.;WANG, Hua;KHANFAR, Mohammad WO2014/100833, 2014, A1 Location in patent:Paragraph 0080; 0232
2976-32-1
46 suppliers
$90.00/500mg

35216-39-8
72 suppliers
$19.00/100mg
20277-69-4
270 suppliers
$10.00/1g
591-19-5
322 suppliers
$10.00/10g

35216-39-8
72 suppliers
$19.00/100mg
3112-85-4
205 suppliers
$15.00/5g

35216-39-8
72 suppliers
$19.00/100mg