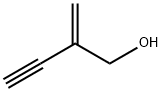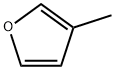
3-Methylfuran synthesis
- Product Name:3-Methylfuran
- CAS Number:930-27-8
- Molecular formula:C5H6O
- Molecular Weight:82.1
4412-96-8
148 suppliers
$16.00/250mg

930-27-8
137 suppliers
$88.79/100mg
Yield: 70%
Reaction Conditions:
with quinoline;copper at 260;
Steps:
12 4.12 Preparation of 3-methylfuran (2c) [71,72]
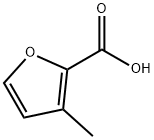
To a stirred Et2O (500mL) solution of 4,4-dimethoxy-2-butanone (80mL, 0.60mol) and methyl chloroacetate (90mL, 1.03mol) was added MeONa (54g, 1.00mol) at -5°C under a N2 atmosphere, and the resulting solution was stirred at -5°C for 2h, and at room temperature overnight. To the solution was slowly added a mixture of glacial acetic acid (7mL) and H2O (93mL) at 0°C. The organic layer was decantated. Aqueous phase was washed with Et2O. The combined organic layers were washed with sat. NaHCO3 aq. and brine, separated, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was distilled (bp 72-78°C/8mmHg) to give 2-methoxycarbonyl-3-methylfuran (71g, 0.51mol, 85% yield). (0033) A mixture of 2-methoxycarbonyl-3-methylfuran (26g, 0.19mol) and 20% NaOH aq. (60mL) was stirred at reflux for 2h. After the solution was cooled to 0°C, conc HCl (35mL) was slowly added to give colorless crystals of 3-methylfuran-2-carboxylic acid (3.199g, 15% yield). (0034) A mixture of 3-methylfuran-2-carboxylic acid (3.20g, 29mmol), Cu (0.90g, 14.2mmol), and quinoline (10mL, 84.4mmol) was heated to 260°C, and a fraction (bp 65.5°C) is collected and assigned to 3-methylfuran (colorless liquid, 65-70% yield) [73].
References:
Maeda, Hajime;Koshio, Norihiro;Tachibana, Yuko;Chiyonobu, Kazuhiko;Konishi, Gen-ichi;Mizuno, Kazuhiko [Journal of Photochemistry and Photobiology A: Chemistry,2017,vol. 349,p. 7 - 17]
498-60-2
253 suppliers
$16.00/1g

930-27-8
137 suppliers
$88.79/100mg
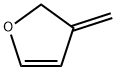
153681-93-7
0 suppliers
inquiry
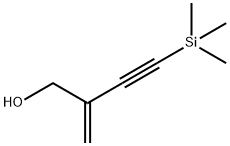
190662-01-2
0 suppliers
inquiry

930-27-8
137 suppliers
$88.79/100mg
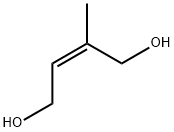
40560-13-2
0 suppliers
inquiry

930-27-8
137 suppliers
$88.79/100mg