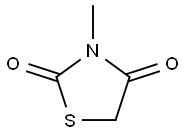
3-METHYL-1,3-THIAZOLANE-2,4-DIONE synthesis
- Product Name:3-METHYL-1,3-THIAZOLANE-2,4-DIONE
- CAS Number:16312-21-3
- Molecular formula:C4H5NO2S
- Molecular Weight:131.15
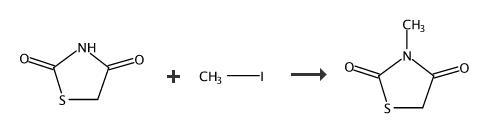
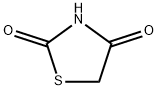
To a solution of 2,4-thiazolidinedione (1 equiv) in 5 mL of DMF was added potassium carbonate (1.1 equiv) and halogen (1.1 equiv). The suspension was stirred at 80 oC overnight. Solvent was evaporated and the residue was purified by flash column chromatography ( petroleum ether/EtOAc, 5:1) to afford the product 3. 3-Methylthiazolidine-2,4-dione (3a) Following the previous procedure, 2,4-thiazolidinedione (500 mg, 4.3 mmol) and iodomethane (0.3 mL, 4.7 mmol). 3a as white solid (460 mg, 82%).
2295-31-0
510 suppliers
$6.00/5g
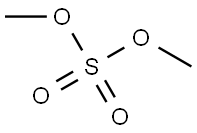
77-78-1
302 suppliers
$22.00/25g

16312-21-3
71 suppliers
$32.00/250mg
Yield:16312-21-3 85%
Reaction Conditions:
Stage #1: thiazolidine-2,4-dionewith potassium carbonate in propan-2-one at 50 - 60; for 0.5 h;
Stage #2: dimethyl sulfate in propan-2-one at 50 - 60; for 12 h;
Steps:
Methylation of thiazolidine-2,4-dione (3):
To a suspension of K2CO3 (2.36 g, 17.1 mmol) in dry acetone (5 mL/1 mmol) 2,4-thiazolidinedione 3 (1 g, 8.54 mmol) was added and the mixture allowed to stir at 50-60 °C for 30 min. Later, Me2SO4 (1.6 mL, 17.1 mmol) was added at the same temperature and the resulting mixture was stirred for another 12 h. After completion of the reaction (TLC), the reaction was stopped by adding water. The reaction mixture was extracted with EtOAc (3 × 20 mL). The combined organic phases were washed with brine, dried over anhydrous Na2SO4, filtered and concentrated. The crude residue was then purified by column chromatography on silica gel with EtOAc/petroleum ether 5:95 afforded N-methylthiazolidine-2,4-dione (5a, 0.95 g, 85%) as a colorless oily liquid.
References:
Kotha, Sambasivarao;Sreevani, Gaddamedi;Dzhemileva, Lilya U.;Yunusbaeva, Milyausha M.;Dzhemilev, Usein M.;D’yakonov, Vladimir A. [Beilstein Journal of Organic Chemistry,2019,vol. 15,p. 2774 - 2781] Location in patent:supporting information

2295-31-0
510 suppliers
$6.00/5g

74-88-4
345 suppliers
$15.00/10g

16312-21-3
71 suppliers
$32.00/250mg
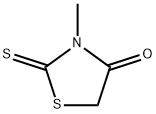
4807-55-0
49 suppliers
$76.29/5g

16312-21-3
71 suppliers
$32.00/250mg
598-52-7
191 suppliers
$6.00/1g
79-11-8
10 suppliers
$27.00/25g

16312-21-3
71 suppliers
$32.00/250mg