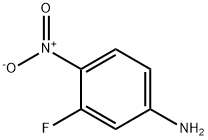
3-Fluoro-4-nitroaniline synthesis
- Product Name:3-Fluoro-4-nitroaniline
- CAS Number:2369-13-3
- Molecular formula:C6H5FN2O2
- Molecular Weight:156.11
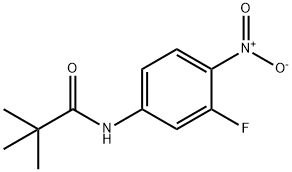
1152311-98-2
1 suppliers
inquiry

2369-13-3
244 suppliers
$19.00/5g
Yield:2369-13-3 99%
Reaction Conditions:
Stage #1:N-(3-fluoro-4-nitro-phenyl)-2,2-dimethyl-propionamide with hydrogenchloride in dichloromethane;water for 2 h;Reflux;
Stage #2: with potassium carbonate in dichloromethane;water;ethyl acetate
Steps:
9.a
A mixture of N-(3-fluoro-4-nitro-phenyl)-2, 2-dimethyl-propionamide (87.0 g, 0.36 mol) in CH2Cl2 (400 mL) and 6N hydrochloric acid (800 mL) was heated to reflux for 2 hours. The reaction mixture was cooled to room temperature. The reaction mixture was diluted with 1000 mL of ethyl acetate and potassium carbonate (500.0 g) was added portion wise. The aqueous solution was separated and the organic layer was washed with brine and dried over anhydrous Na2SO4. The solvent was removed by evaporation under reduced pressure; the residue was purified by column chromatography on silica gel (petroleum ether / ethyl acetate 30: 1) to afford 3-fluoro-4-nitroaniline (56.0 g, 99 %). 1H NMR (300 MHz, CDCl3) δ 8.07 (t, J = 8.7 Hz, 1 H), 7.86 (dd, J= 2.1, 13.2 Hz 1 H), 7.59 (brs, 2 H), 7.22 (s, 1 H).
References:
VERTEX PHARMACEUTICALS INCORPORATED;RUAH, Sara, Hadida, S.;GROOTENHUIS, Peter, D., J.;VAN GOOR, Fredrick;MILLER, Mark, T.;MCCARTNEY, Jason;ZHOU, Jinglan;BEAR, Brian;NUMA, Mehdi, Michel, Djamel WO2010/53471, 2010, A1 Location in patent:Page/Page column 56
372-19-0
322 suppliers
$10.00/1g

2369-13-3
244 suppliers
$19.00/5g
403-21-4
253 suppliers
$6.00/1g
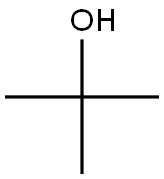
75-65-0
753 suppliers
$10.00/10ml
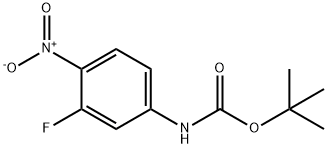
658700-15-3
9 suppliers
inquiry

2369-13-3
244 suppliers
$19.00/5g

658700-15-3
9 suppliers
inquiry

2369-13-3
244 suppliers
$19.00/5g
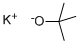
865-47-4
677 suppliers
$14.00/25g
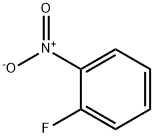
1493-27-2
512 suppliers
$5.00/10g
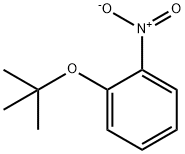
83747-12-0
13 suppliers
inquiry

2369-13-3
244 suppliers
$19.00/5g
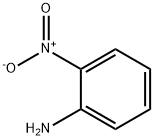
88-74-4
7 suppliers
$10.00/1g