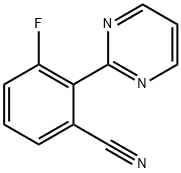
3-fluoro-2-(pyrimidin-2-yl)benzonitrile synthesis
- Product Name:3-fluoro-2-(pyrimidin-2-yl)benzonitrile
- CAS Number:1293285-05-8
- Molecular formula:C11H6FN3
- Molecular Weight:199.18
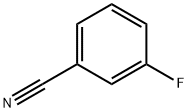
403-54-3
337 suppliers
$7.00/5g
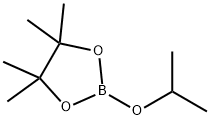
61676-62-8
327 suppliers
$11.19/5G
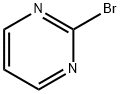
4595-60-2
357 suppliers
$8.00/5g

1293285-05-8
5 suppliers
inquiry
Yield:1293285-05-8 118 g
Reaction Conditions:
Stage #1: m-Fluorobenzonitrile;2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolanewith lithium diisopropyl amide in tetrahydrofuran at -78 - -71; for 1.75 h;
Stage #2: 2-bromopyrimidinewith dichloro[1,1′-bis[bis(1,1-dimethylethyl)phosphino]ferrocene-P,P′]palladium at 66; for 1 h;Inert atmosphere;
Steps:
238.A Step A: 3-fluoro-2-(pyrimidin-2-yl)benzonitrile
j0946] To a i 2-E 4-necked round-bottomed flask equipped with a thermocouple probe, mechanical stirrer, condenser and nitrogen inlet was charged 3-fluorobenzonitrile (i40 g, i23.6 mmol), 2-isopropoxy-4,4,5,5-tetramethyl- i ,3,2-dioxaboro- lane (353.7 mE, i.699 mol), and THF (2.35 E). The mixture was cooled to -78°C. and lithium diisopropylamide (623 mE, i .246 mol, 2 M) was added over 45 mm maintaining a temperature of <-7 i ° C. The mixture was stirred for i hat -76° C. then quenched with sodium bicarbonate(0q) (i72 gin i 500 mE water). This mixture was warmed to room temperature to produce an off-white slurry. The slurry was treated with 2-bromopyrimidine (i7i.8 g, i.059 mol) and then degassed with bubbling nitrogen. Dichloro[i , i ‘-bis(di-t-butylphosphino)ferrocene]palladium(II) (i7 g, 25.8 mmol) was then added and the mixture was heated to 66° C. for i h. The mixture was cooled and ethyl acetate (5.6 E) was added. Solids were removed by filtration and washed with ethyl acetate (2x300 mE). The layers were cut and the aqueous layer was extracted with ethyl acetate (2 E). The combined organic layers were washed with brine (2x i .2 E) and then concentrated. Ethanol (600 mE) was added and the mixture was further concentrated to provide a dark brown liquid (382.0 g, 96% mass recovery, 75.5% desired, i9.i% regioisomer (3-fluoro-4-(pyrimidin-2-yl)benzonitrile). This liquid was warmed in ethanol (600 mE) at 66° C. until homogeneous and then gradually cooled to 20° C. The resulting solids were isolated by filtration and washed with cold i/i hexanes/ethanol (2xiOO mE). After drying for 3 hours under air suction, the title compound was obtained as an off-white solid (ii 8 g, 30%, 99.2% desired regioisomer). The mother liquor contained 20% additional desired product that could be recovered through chromatography and crystallization. ‘H NMR (400 MHz, CDC13) ? 8.97 (d, J=4.9 Hz, 2H), 7.69-7.6i (m, iH), 7.6i-7.52 (m, iH), 7.5i-7.43 (m, iH), 7.4i (t, J=5.0 Hz, iH).
References:
US2016/46640,2016,A1 Location in patent:Paragraph 0946
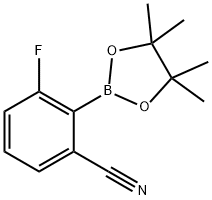
624741-47-5
47 suppliers
$28.00/100mg

4595-60-2
357 suppliers
$8.00/5g

1293285-05-8
5 suppliers
inquiry
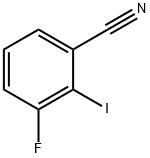
916792-62-6
93 suppliers
inquiry
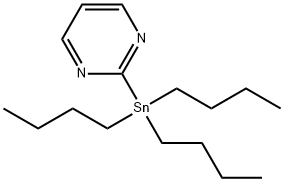
153435-63-3
111 suppliers
$35.00/250mg

1293285-05-8
5 suppliers
inquiry