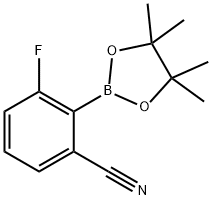
3-Fluoro-2-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzonitrile synthesis
- Product Name:3-Fluoro-2-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzonitrile
- CAS Number:624741-47-5
- Molecular formula:C13H15BFNO2
- Molecular Weight:247.07
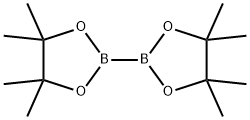
73183-34-3
591 suppliers
$6.00/5g
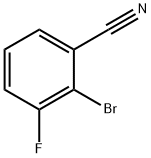
425379-16-4
114 suppliers
inquiry

624741-47-5
47 suppliers
$28.00/100mg
Yield:624741-47-5 62%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane;dimethyl sulfoxide at 90; for 18 h;
Steps:
2.a
A mixture of 2-bromo-3-fluorobenzonitrile (1.20 g, 6.00 mmol), dried potassium acetate (1.18 g, 12.0 mmol) and bis (pinacolato) diboron (1.75 g, 6.89 mmol) in 1, 4-dioxan (14.7 ml) and dimethylsulfoxide (0.3 ml) was degassed by bubbling nitrogen through the mixture for 50 min. Dichloro [l, 1'-bis (diphenylphosphino) ferrocene] palladium (II) dichloromethane adduct (0.1481 g, 0.181 mmol) was added and the mixture was degassed for a further 10 min, then heated at 90°C under nitrogen for 18 h. After allowing to cool, the mixture was filtered through glass fibre paper, and the solid was washed with a little dichloromethane. The combined filtrates were evaporated in vacuo and the residue was partitioned between 2 M aqueous NaOH (21 ml) and diethyl ether (20 ml). The aqueous layer was then acidified to pH 5 with concentrated hydrochloric acid (3 ml), causing a solid to precipitate. After leaving in a fridge for 3 days, the solid was collected by filtration, washed with water and dried under vacuum to leave 0.9191 g (62%) of the title compound as a white solid: 1H NMR (360 MHz, DMSO-d6) 8 1.34 (12H, s), 7.55-7. 59 (1H, m), 7.69-7. 75 (2H, m).
References:
WO2003/93272,2003,A1 Location in patent:Page/Page column 37