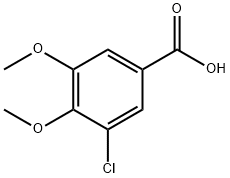
3-CHLORO-4 5-DIMETHOXYBENZOIC ACID 97 synthesis
- Product Name:3-CHLORO-4 5-DIMETHOXYBENZOIC ACID 97
- CAS Number:20624-87-7
- Molecular formula:C9H9ClO4
- Molecular Weight:216.62
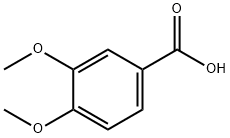
93-07-2
570 suppliers
$5.00/5g

20624-87-7
28 suppliers
$60.00/50mg
Yield:-
Reaction Conditions:
with sulfuryl dichloride at 50; for 24 h;
Steps:
General procedure: SO2Cl2 (10 mL) and 4-methoxybenzoic acid (5.0 g) was added to the reaction bulb, and then stirred at 50°C for 24 h. After concentration under reduced pressure, the residue was re-crystallized from boiling water to give 3-chloro-4,5-dimethoxybenzoic acid 5a as white needles. To perform bromide of methoxybenzoic acid, we used Br2 in CCl4. A solution of 4-methoxybenzoic acid 5a (1.0 g) and DMF (catalytic amount) in thionyl chloride (5 mL) was stirred at 80-90°C for 2h. After reclaimed the thionyl chloride under reduced pressure, 4-methoxybenzoyl chloride 6a was yielded. 4-methoxybenzoyl chloride 6a was dissolved in dried CH2Cl2 and reacted with excess furan (5mL) catalyzed by AlCl3. The reaction mixture was stirred at 0°C for 2 h and then warmed to room temperature and stirred for additional 12 h. After the reaction was completed, the reaction was quenched by carefully pouring the mixture into iced water (100 mL) and the resultant compound was collected by filtration. Then, the filtrate was extracted with CH2Cl2 (320mL), and dried with anhydrous Na2SO4. The solvent was removed under reduced pressure to yield brown solid. The brown solid was purified by silica gel column chromatography (Petroleum ether/EtOAc 80:20, v/v) to give compound 7a as light yellow solid. 10% (eq) BBr3 (5 mL) was added to a solution of compound 7a (0.5 g) in CH2Cl2 (20 mL). The reaction mixture was stirred at -78°C for 30 min and then warmed to room temperature and stirred for additional 3.5 h. After the reaction was completed, it was quenched by carefully pouring the mixture into iced water (100 mL), extraction of the aqueous layer three times with EtOAc, washing with 5% NaHSO3 (40 mL) and water (100 mL), and drying with anhydrous Na2SO4. The solvent was removed under reduced pressure to yield a light red solid 8a (0.551 g, 50% yield).
References:
Zheng, Fei Lang;Ban, Shu Rong;Feng, Xiu E.;Zhao, Cheng Xiao;Du, Guan Hua;Li, Qing Shan [Medicinal Chemistry,2013,vol. 9,# 2,p. 303 - 311]
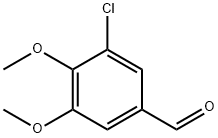
18268-68-3
45 suppliers
$20.00/250mg

20624-87-7
28 suppliers
$60.00/50mg
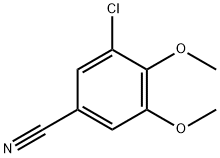
90537-30-7
18 suppliers
$94.70/5g

20624-87-7
28 suppliers
$60.00/50mg