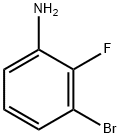
3-Bromo-2-fluoroaniline synthesis
- Product Name:3-Bromo-2-fluoroaniline
- CAS Number:58534-95-5
- Molecular formula:C6H5BrFN
- Molecular Weight:190.01
3-Bromo-2-fluoroaniline is a biochemical used in the synthesis of 4-amino-3-fluorophenyl boronic acid. It was also used in chemical modification and synthesis of boronic acid derivatives for ultraviolet-visible titration. Under argon, 3-bromo-2-fluoronitrobenzene was initially charged in dioxane, and tin(II) chloride and a few drops of 1 N hydrochloric acid were added. After 2 h of stirring, the reaction mixture was purified to gave 3-Bromo-2-fluoroaniline.
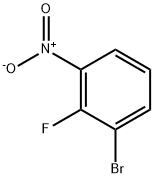
58534-94-4
186 suppliers
$10.00/1g

58534-95-5
178 suppliers
$8.00/1g
Yield:58534-95-5 95%
Reaction Conditions:
Stage #1:2-fluoro-3-bromonitrobenzene with tin(ll) chloride;hydrogenchloride in 1,4-dioxane for 2 h;Inert atmosphere;
Stage #2: with sodium hydroxide in water
Steps:
227A
Under argon, 1.0 g (4.54 mmol) of 3-bromo-2-fluoronitrobenzene was initially charged in 5 ml of dioxane, and 4.3 g (22.72 mmol) of tin(II) chloride and a few drops of 1 N hydrochloric acid were added. After 2 h of stirring, the reaction mixture was concentrated on a rotary evaporator and the residue was taken up in ethyl acetate. The organic phase was washed with 1 N aqueous sodium hydroxide solution, water and saturated sodium chloride solution and then dried over magnesium sulfate and concentrated. This gave 826 mg (95% of theory) of the title compound.LC-MS (method 15): Rt=0.89 min; m/z=190 (M+H)+.1H-NMR (400 MHz, DMSO-d6): δ=6.83-6.70 (m, 3H), 5.43 (s, 2H).
References:
BAYER SCHERING PHARMA AKTIEGESELLSCHAFT US2011/34450, 2011, A1 Location in patent:Page/Page column 95
848440-23-3
11 suppliers
inquiry

58534-95-5
178 suppliers
$8.00/1g

161957-56-8
275 suppliers
$6.00/1g

58534-95-5
178 suppliers
$8.00/1g