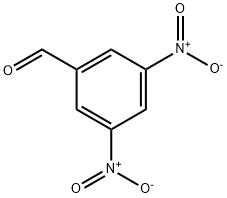
3,5-DINITROBENZALDEHYDE synthesis
- Product Name:3,5-DINITROBENZALDEHYDE
- CAS Number:14193-18-1
- Molecular formula:C7H4N2O5
- Molecular Weight:196.12
Yield: 45.8 g (89%)
Reaction Conditions:
with hydrogenchloride;sodium hydrogencarbonate;triethylamine in tetrahydrofuran;dichloromethane;water;dimethyl sulfoxide
Steps:
83 (3,5-Dinitrobenzaldehyde)
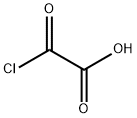
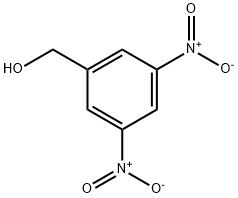
EXAMPLE 83 (3,5-Dinitrobenzaldehyde) To a solution of oxalylchloride (26 ml, 0.30 mol) in CH2Cl2 (600 ml) DMSO (43 ml, 0.60 mol) was added, maintaining the temperature between -50° C. and -65° C. To the reaction solution was then added 3,5-dinitrobenzyl alcohol (50g), in THF (100 ml), maintaining the temperature between -50° C. and -65° C. The reaction mixture was stirred at -65° C. for 30 minutes and then triethylamine (170 ml) was added at -50° C. and -65° C. The reaction temperature was then adjusted to room temperature and H2O (800 ml) was added. The organic phase was washed with sat. NaHCO3 (500 ml), 5 times with diluted HCl (300 ml) and finally brine. The organic phase was then dried over MgSO4 and the volatile solvents were removed at reduced pressure. The crude product was then triturated with heptane, which gave 45.8 g (89%) of 3,5-dinitrobenzaldehyde. 1H NMR (CDCl3, 300 MHz) δ ppm: 10.22 (s, 1H), 9.30 (d, 1H), 9.05 (t, 2H).
References:
Amersham Health AS US6448442, 2002, B1
71022-43-0
151 suppliers
$25.00/1g

14193-18-1
57 suppliers
inquiry
99-33-2
3 suppliers
$12.00/5g

14193-18-1
57 suppliers
inquiry
99-34-3
535 suppliers
$14.00/5g

14193-18-1
57 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

14193-18-1
57 suppliers
inquiry