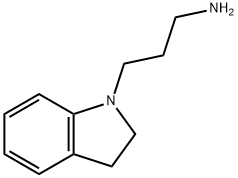
3-(2,3-DIHYDRO-1H-INDOL-1-YL)PROPAN-1-AMINE synthesis
- Product Name:3-(2,3-DIHYDRO-1H-INDOL-1-YL)PROPAN-1-AMINE
- CAS Number:61123-70-4
- Molecular formula:C11H16N2
- Molecular Weight:176.26
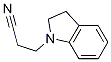
91349-96-1
7 suppliers
inquiry

61123-70-4
20 suppliers
$45.00/25mg
Yield:61123-70-4 68%
Reaction Conditions:
with lithium aluminium tetrahydride in diethyl ether at 0 - 20; for 24 h;
Steps:
Procedure E:
General procedure: Reduction of a nitrile (Amundsen et al., 1951). To a solution of a nitrile (n g) in anhydrous diethyl ether (60 mL) at 0°C was slowly added LAH (n g). The resulting mixture was allowed to warm slowly to room temperature and was further stirred for 24 h. To the mixture was then added water n mL, n mL of 15% NaOH and 3n mL of water. The ether solution was filtered through celite and evaporated.Procedure E: Reduction of a nitrile (Amundsen et al., 1951). To a solution of a nitrile (n g) in anhydrous diethyl ether (60 mL) at 0°C was slowly added LAH (n g). The resulting mixture was allowed to warm slowly to room temperature and was further stirred for 24 h. To the mixture was then added water n mL, n mL of 15% NaOH and 3n mL of water. The ether solution was filtered through celite and evaporated. ;3-(Indolin-1-yl)propan-1-amine, (9a). (a) Petrovna et al., 2010; b) Shapiro et al., 1959). Compound 9a synthesised from 8a by Procedure E, was isolated as a yellow oil in 68% yield. 1H-NMR (300 MHz, CDC13) ppm 6.90-6.86 (m, 2H), 6.48 (t, J = 7.2 Hz, 1H), 6.29 (t, J= 7.2 Hz, 1H), 3.09 (t, J= 8.1 Hz, 1H), 2.87 (t, J= 6.9 Hz, 1H), 2.74 (t, J= 8.1 Hz, 2H), 2.50 (t, J = 6.9 Hz, 2H), 1.47 (t, J = 7.2 Hz, 2H); 13C-NMR (75 MHz, CDC13) ppm 152.02, 129.84, 126.75, 124.02, 116.99, 106.43, 52.63, 48.44, 39.43, 30.42, 28.03; MS (EIj m/z 176.134 (M); HRMS calcd. for C11H16N2 (M, EIj 176.1313 found176.1337.
References:
WO2017/125932,2017,A1 Location in patent:Page/Page column 34; 35; 49
496-15-1
422 suppliers
$8.00/10g

5003-71-4
375 suppliers
$5.00/10g

61123-70-4
20 suppliers
$45.00/25mg
4414-79-3
4 suppliers
inquiry

61123-70-4
20 suppliers
$45.00/25mg
120-72-9
631 suppliers
$6.00/25g

61123-70-4
20 suppliers
$45.00/25mg

496-15-1
422 suppliers
$8.00/10g

14753-26-5
20 suppliers
inquiry

61123-70-4
20 suppliers
$45.00/25mg