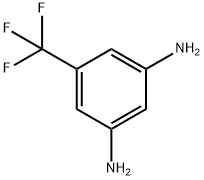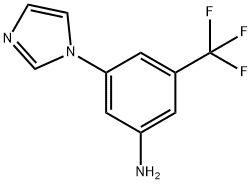
3-(1H-imidazol-1-yl)-5-(trifluoromethyl)aniline synthesis
- Product Name:3-(1H-imidazol-1-yl)-5-(trifluoromethyl)aniline
- CAS Number:943320-48-7
- Molecular formula:C10H8F3N3
- Molecular Weight:227.19
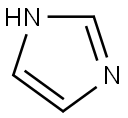
288-32-4
1010 suppliers
$5.00/25g
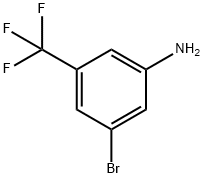
54962-75-3
300 suppliers
$5.00/1g

943320-48-7
33 suppliers
inquiry
Yield:943320-48-7 63%
Reaction Conditions:
with copper(l) iodide;caesium carbonate in N,N-dimethyl-formamide at 20 - 130;Reagent/catalyst;Solvent;
Steps:
1.2; 2.2; 3.2; 4.2 Step 2: Preparation of 3-(1H-imidazol-1-yl)-5-trifluoromethylaniline
The 3-bromo-5-trifluorobenzylaniline (2g, 0.0084mol) prepared in step 1 was dissolved in 15mL solution of DMF. Add 2.5g of cesium carbonate (0.0076mol), imidazole (1.16g, 0.0235mol), cuprous iodide (0.16g, 0.00084mol), stir at room temperature for 10min, then heat the reaction system to 130 and stir for 16-20h, TLC monitors the reaction Complete, cool to room temperature, filter, add 15mL water to the filtrate, stir at room temperature for 30min, add ethyl acetate to extract 4 times (15mL*4) and wash the organic phase 4 times with water, separate the organic phase, dry and concentrate the organic phase to obtain 1.78g crude product. The crude product is purified by column chromatography, the eluent DCM:MeOH is 50:1-20:1 (gradient elution: DCM:MeOH is stirred evenly at a ratio of 50:1 to separate the less polar impurities, the elution process Using TLC monitoring in the medium, when the impurity disappears, the target product is separated evenly by stirring according to the ratio of 20:1) to obtain 1.2 g of 3-imidazol-1-yl-5-trifluoromethylaniline as a pale yellow solid, and the yield is 63%.
References:
CN112778207,2021,A Location in patent:Paragraph 0039; 0048; 0051-0055; 0058-0060; 0063-0065; 0068