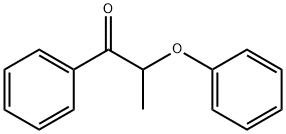
2-PHENOXYPROPIOPHENONE synthesis
- Product Name:2-PHENOXYPROPIOPHENONE
- CAS Number:6640-19-3
- Molecular formula:C15H14O2
- Molecular Weight:226.27
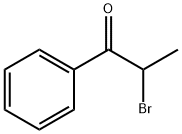
2114-00-3
7 suppliers
$15.00/5g
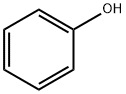
108-95-2
739 suppliers
$14.00/25g

6640-19-3
5 suppliers
$90.00/500mg
Yield:6640-19-3 85%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 24 h;Inert atmosphere;Williamson Ether Synthesis;
Steps:
Synthesis of α-oxyketones 1d-p, 1s-w and 6
General procedure: General experimental procedure B (GEP-B): To a clean and oven-dried flask 1 equiv of the corresponding α-bromo ketone SM2-6 or desyl chloride (SM1), anhydrous DMF, 1.1-1.3 equiv of K2CO3 and 1.1-1.3 equiv of the corresponding phenol were added under nitrogen atmosphere. The mixture was allowed to react for 24 h at r.t. The reaction was worked up with H2O (15 mL) and extracted with Et2O (3 × 15 mL). The organic layers were combined, washed with aqueous NaOH (2 × 20 ml, 10% w/w) and dried over anhydrous Na2SO4. After evaporation under reduced pressure, the crude residue was purified by flash column chromatography on silica gel (eluent: hexane/EtOAc) to obtain α-oxy ketones 1 and 6.
References:
Sedano, Carlos;Virumbrales, Cintia;Suárez-Pantiga, Samuel;Sanz, Roberto [Synthesis,2021,vol. 53,# 20,p. 3725 - 3734] Location in patent:supporting information
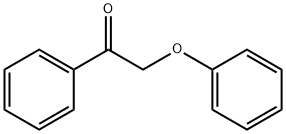
721-04-0
61 suppliers
$60.00/100mg

74-88-4
347 suppliers
$15.00/10g

6640-19-3
5 suppliers
$90.00/500mg

67-56-1
752 suppliers
$9.00/25ml

721-04-0
61 suppliers
$60.00/100mg

6640-19-3
5 suppliers
$90.00/500mg
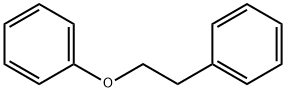
40515-89-7
79 suppliers
$10.00/1g

6640-19-3
5 suppliers
$90.00/500mg

108-95-2
739 suppliers
$14.00/25g

6640-19-3
5 suppliers
$90.00/500mg