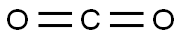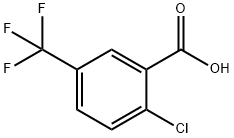
2-Chloro-5-(trifluoromethyl)benzoic acid synthesis
- Product Name:2-Chloro-5-(trifluoromethyl)benzoic acid
- CAS Number:657-06-7
- Molecular formula:C8H4ClF3O2
- Molecular Weight:224.56
Yield:657-06-7 59%
Reaction Conditions:
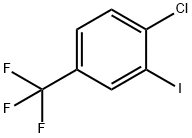
Stage #1: 4-chlorobenzotrifluoridewith n-butyllithium;N,N,N,N,-tetramethylethylenediamine in tetrahydrofuran;hexanes at -78; for 1 h;Inert atmosphere;
Stage #2: carbon dioxide in tetrahydrofuran;hexanes; for 0.333333 h;
Stage #3: in water; pH=1;Acidic aqueous solution;
Steps:
Preparation of 2-chloro-5-trifluoromethylbenzoic acid [Show Image]n-BuLi (2.5M in hexanes, 22 mL, 55 mmol) was added over 8 minutes to a stirred solution of 4-chlorobenzotrifluoride (9.177 g, 50.8 mmol) and TMEDA (6.3030 g, 54.2 mmol) in THF (89 mL) at -78°C under nitrogen. After 52 min the solution was transferred via cannula into dry ice (~200g) over 20 min. Care was required to sufficiently lag the cannula as the anion solution was found to darken rapidly on slight warming. The mixture was allowed to warm to ambient temperature with stirring and was evaporated (<30°C) to give and orange/yellow solid. The solid was dissolved in water (70 mL) and washed with diethyl ether (3x30 mL). The aqueous layer was acidified to pH 1 and extracted with DCM (3x30 mL). The organic layer was evaporated to dryness (< 30°C) and the residue was dissolved in refluxing hexanes, hot filtered and crystallised by cooling to 4°C overnight. The sandy coloured solid was isolated by vacuum filtration (4.5243 g). The mother liquors were concentrated by half and a second crop of lemon yellow crystals was obtained (2.2998 g). The products were dried under vacuum to give 4.5030 g and 2.2093 g respectively, giving a combined yield of 6.712 g (59 %). LCMS: Rt = 5.94 min, first crop = 96.3 A% (at) 254 nm, second crop 95.1 A%, [M+H]+ 225.0, with no starting material detected.
References:
EP2468746,2012,A1 Location in patent:Page/Page column 6
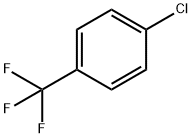
98-56-6
395 suppliers
$11.00/25g

657-06-7
191 suppliers
$7.00/1g
121-50-6
331 suppliers
$5.00/5g

657-06-7
191 suppliers
$7.00/1g