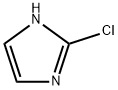
2-Chloro-1H-imidazole synthesis
- Product Name:2-Chloro-1H-imidazole
- CAS Number:16265-04-6
- Molecular formula:C3H3ClN2
- Molecular Weight:102.52
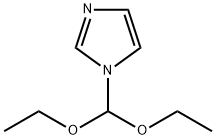
61278-81-7
35 suppliers
$272.00/10ml

16265-04-6
147 suppliers
$14.00/100mg
Yield:16265-04-6 85%
Reaction Conditions:
Stage #1: 1-(diethoxymethyl)-1H-imidazolewith n-butyllithium in tetrahydrofuran;hexane at -35;
Stage #2: with hexachloroethane in tetrahydrofuran;hexane at -35; for 0.0833333 h;
Stage #3: with hydrogenchloride;sodium hydroxidemore than 3 stages;
Steps:
9 Preparation of 2-chloroimidazole
Reference example 9 Preparation of 2-chloroimidazole N-(Diethoxymethyl)imidazole (50.0 g) was dissolved in tetrahydrofuran (200 ml), to this solution was added dropwise n-hexane solution (120 ml) of 2.6M n-butyllithium at lower than -35°C, next tetrahyfrofuran solution (100 ml) of hexachloroethane (73.9 g) was added dropwise. Reaction mixture was allowed to stand at the same temperature for 5 minutes, then temperature was rised, after being added 6N hydrochloric acid (100 ml) at -20°C, then turned back to room temperature, and allowed to stand for 5 minutes. The aqueous layer was taken by separation, and the organic layer was extracted with 1N hydrochloric acid, the extract was combined with the former aqueous layer, and washed with diethyl ether, then neutralized with an aqueous solution of 6N sodium hydroxide, and extracted with ethyl acetate. The organic layer was taken by separation, after being dried over anhydrous magnesium sulfate, the solvent was removed by distillation to obtained a crude product. This crude product was triturated with methylene chloride, there was obtained 2-chloroimidazole (26.0 g, yield: 85.0%) as pale brown solid product. 1H-NMR (CDCl3) δ (ppm): 10.64 (1H, bs), 7.05 (1H, s).
References:
EP1553088,2005,A1 Location in patent:Page/Page column 52
15469-97-3
206 suppliers
$9.00/250mg

16265-04-6
147 suppliers
$14.00/100mg
288-32-4
1010 suppliers
$5.00/25g

16265-04-6
147 suppliers
$14.00/100mg