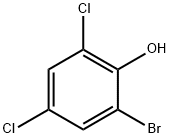
2-bromo-4,6-dichlorophenol synthesis
- Product Name:2-bromo-4,6-dichlorophenol
- CAS Number:4524-77-0
- Molecular formula:C6H3BrCl2O
- Molecular Weight:241.9
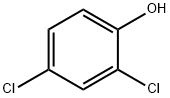
120-83-2
395 suppliers
$12.90/100g

4524-77-0
62 suppliers
$199.00/500mg
Yield:4524-77-0 97%
Reaction Conditions:
Stage #1: 2,4-dichlorophenolwith bromine in toluene at -50; for 0.333333 h;
Stage #2: with tert-butylamine in toluene at 50; for 0.5 h;
Steps:
Synthesis of 2-bromo-4,6-dichlorophenol (2):
To a solution of 2,4-dichlorophenol (1, 20.0 g, 0.12 mol) in toluene (200 ml), bromine (22 ml, 0.24 mol) was added at -50 °C and stirred for 20 min. To this reaction, t-butylamine (13 ml, 0.12 mol) was added at -50 °C and stirred for 30 min. The reaction mixture was quenched with aqueous sodium thiosulphate (300 ml) and the organic layer was separated. The aqueous layer was extracted with hexane (3 x 100 ml). The organic layers were combined, dried (Na2SO4) and concentrated in vacuo to give 2-bromo-4,6-dichlorophenol (2, 28.0 g, 97%) as an off-white solid. MS (ESI +ve): 241 1H-NMR (400 MHz; CDCl3): d 5.84 (s, 1H), 7.32 (s, 1H), 7.42 (s, 1H).
References:
O'Brien, Alistair;Andrews, Stephen P.;Baig, Asma H.;Bortolato, Andrea;Brown, Alastair J.H.;Brown, Giles A.;Brown, Sue H.;Christopher, John A.;Congreve, Miles;Cooke, Robert M.;De Graaf, Chris;Errey, James C.;Fieldhouse, Charlotte;Jazayeri, Ali;Marshall, Fiona H.;Mason, Jonathan S.;Mobarec, Juan Carlos;Okrasa, Krzysztof;Steele, Kelly N.;Southall, Stacey M.;Teobald, Iryna;Watson, Steve P.;Weir, Malcolm [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 20,art. no. 126611] Location in patent:supporting information
108-95-2
739 suppliers
$14.00/25g

4524-77-0
62 suppliers
$199.00/500mg
2446-83-5
495 suppliers
$12.00/1g

120-83-2
395 suppliers
$12.90/100g
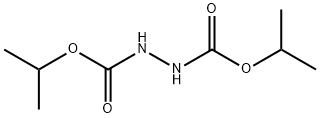
19740-72-8
106 suppliers
$16.00/100mg

4524-77-0
62 suppliers
$199.00/500mg
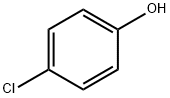
106-48-9
483 suppliers
$10.00/5G

4524-77-0
62 suppliers
$199.00/500mg
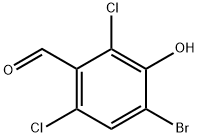
873983-87-0
1 suppliers
inquiry

4524-77-0
62 suppliers
$199.00/500mg