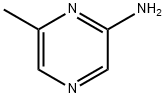
2-Amino-6-methylpyrazine synthesis
- Product Name:2-Amino-6-methylpyrazine
- CAS Number:5521-56-2
- Molecular formula:C5H7N3
- Molecular Weight:109.13
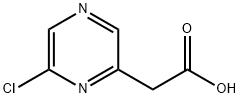
930798-25-7
84 suppliers
$72.00/250mg

5521-56-2
141 suppliers
$33.00/200mg
Yield: 71%
Reaction Conditions:
with ammonia in water at 180; under 26252.6 Torr; for 8 h;sealed vessel;
Steps:
1.c
60ml) at O0C was added, diethylmalonate (2.1moleq) in THF (60ml) and 2,6- dichloropyrazine (2Og) in THF (40ml). The mixture was then heated to reflux for 18hrs before being allowed to cool and 2M Hydrochloric acid (100ml) added. The resulting two layers were separated and the THF layer partially concentrated under vacuum to give a solution containing 2-(6-chloro-pyrazin-2-yl)-malonic acid diethyl ester. This solution was EPO
References:
ASTRAZENECA AB WO2007/35153, 2007, A1 Location in patent:Page/Page column 2; 4-5
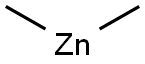
544-97-8
132 suppliers
$130.00/5g
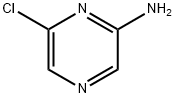
33332-28-4
297 suppliers
$9.00/1g

5521-56-2
141 suppliers
$33.00/200mg
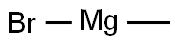
75-16-1
269 suppliers
$12.00/10ml

33332-28-4
297 suppliers
$9.00/1g

5521-56-2
141 suppliers
$33.00/200mg
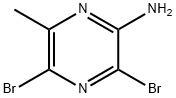
74290-66-7
68 suppliers
$76.00/250mg

5521-56-2
141 suppliers
$33.00/200mg
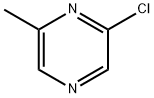
38557-71-0
174 suppliers
$5.00/250mg

5521-56-2
141 suppliers
$33.00/200mg