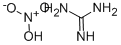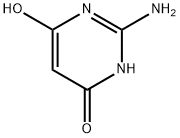
2-Amino-4,6-dihydroxypyrimidine synthesis
- Product Name:2-Amino-4,6-dihydroxypyrimidine
- CAS Number:56-09-7
- Molecular formula:C4H5N3O2
- Molecular Weight:127.1
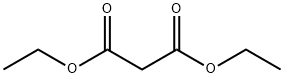
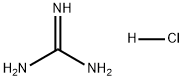
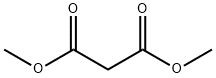
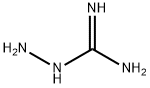
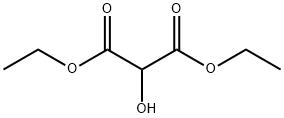
A freshly-prepared sodiummethoxide solution in methanol (25 ml) was added to a round-bottomed flask containing guanidine hydrochloride(15 mmol), stirred for a few minutes, before dimethyl malonate (15 mmol)was slowly added. The mixture was heated under reflux for about 2-3hours. After evaporation of the solvent under reduced pressure, the resultingwhite solid was dissolved in a minimum amount of water and the pH adjusted to 6with 10% HCl. The precipitate obtained was filtered and washed with distilledwater and ethanol, respectively, to obtain pure 2-amino-4,6-pyrimidinediol.(Note: sodium methoxide solution was prepared by addition of small pieces ofsodium metal in methanol).Yield: 85%;solid, m.p. 195-197 °C.
Yield:56-09-7 96.1%
Reaction Conditions:
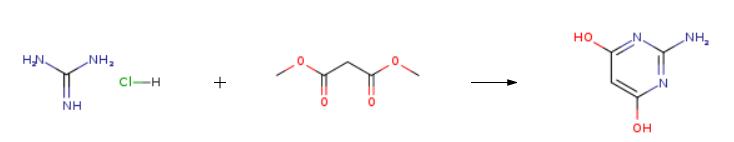
Stage #1: guanidine nitratewith sodium methylate in ethanol at 5; for 0.5 h;
Stage #2: diethyl malonate in ethanol at 65; for 6 h;Solvent;Temperature;
Steps:
1; 2; 3 The first step, 2-amino-pyrimidine-4,6-diol Synthesis of
Temperature 5 , into 30.52g (0.25mol) of guanidine nitrate, and 100ml of anhydrous ethanol 500ml 4-neck flask, stirred open, the ethanol solution was slowly added dropwise 250ml 2.5M sodium methoxide After the solid was completely dissolved, stirring incubated 0.5 H; then slowly added dropwise to the reaction flask 41.64g (0.26mol) of diethyl malonate dropwise, the reaction temperature was raised to 65 deg.] C 6h.After completion of the reaction, concentrated under reduced pressure to give an off-white solid.After drinking water 60ml was added to the reaction flask and the solid was dissolved, washed with 10% dilute hydrochloric acid solution in the system was adjusted to pH 6, and the precipitated solid was large; suction filtered and rinsed with water, pressed dry, wet cake at 60 blast drying to constant weight, to give 30.56 g white product, this step a yield of 96.10%, HPLC purity of 99.7%.
References:
CN109456329,2019,A Location in patent:Paragraph 0022-0024