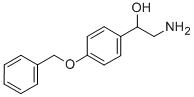
2-AMINO-1-(4'-BENZYLOXYPHENYL)ETHANOL synthesis
- Product Name:2-AMINO-1-(4'-BENZYLOXYPHENYL)ETHANOL
- CAS Number:56443-72-2
- Molecular formula:C15H17NO2
- Molecular Weight:243.3
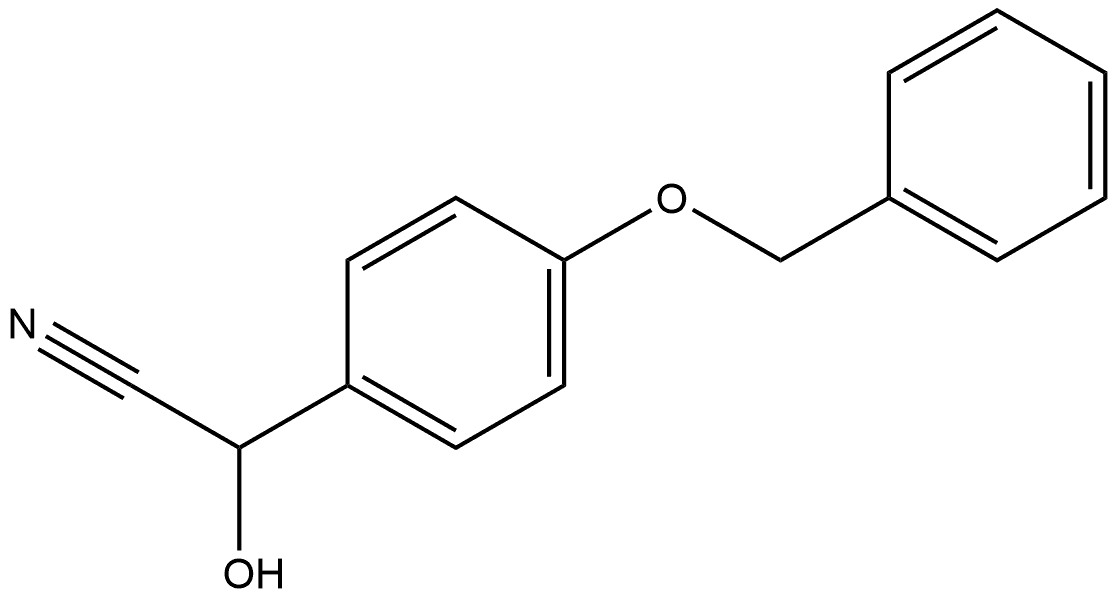
58327-40-5
3 suppliers
inquiry

56443-72-2
19 suppliers
$125.09/10mg
Yield:56443-72-2 73%
Reaction Conditions:
Stage #1: α-Hydroxy-4-(phenylmethoxy)benzeneacetonitrilewith dimethylsulfide borane complex in tetrahydrofuran; for 2 h;Heating / reflux;
Stage #2: with hydrogenchloride in water; for 2 h;
Stage #3: with ammonia in water;
Steps:
24 EXAMPLE 24; 2-AMINO-1- (4-BENZYLOXYPHENVL) ETHANOL
Potassium cyanide (20.15g, 0. 31mol) and ammonium CHLORIDE (16. 4g, 0. 31mol) were dissolved in water (60ml) to which was added 4-benzyloxybenzaldehyde (32.9g, 0. 155mol) followed by diethyl ether (100MOL). The reaction mixture was stirred vigorously for 48 hours at room temperature before extracting with ethyl acetate (2 x 200ml). The combined organic layers were dried over anhydrous magnesium sulphate, filtered and concentrated in vacuo to give the cyanohydrin intermediate as a yellow solid (34.2g, 0. 14MOL, 90%). The cyanohydrin was then dissolved in DRY THF (300ML) and borane-methyl sulphide complex (26. 6MOI, 0. 28MOL) was added. The reaction mixture was REFLUXED for 2 hours before being quenched with methanol (50ml). Water (50ml) was added followed by C. HCI (40ML) and the reaction mixture was stirred for 2 hours until the exotherm subsided. The reaction mixture was then concentrated in vacuo and the residue diluted with water (100ml). The aqueous solution was then basified by addition of NH40H (30ml), and extracted with ethyl acetate (3 x 150ml). The organic extracts were dried over anhydrous magnesium sulphate, filtered and concentrated in vacuo to give the title compound as a white solid (24.8g, 0. 10MOL, 73%). 1H NMR (CDCI3, 400MHZ) 6 : 1.62 (bs, 3H), 2.81 (dd, 1 H), 2.99 (d, 1 H), 4.61 (q, 1 H), 5.07 (s, 2H), 6.95 (d, 2H), 7.22-7. 45 (m, 7H) LRMS: M/Z 244 (M-H+).
References:
WO2004/52372,2004,A1 Location in patent:Page 76-77
![Ethanone, 2-azido-1-[4-(phenylmethoxy)phenyl]-](/CAS/20180601/GIF/831196-83-9.gif)
831196-83-9
0 suppliers
inquiry

56443-72-2
19 suppliers
$125.09/10mg
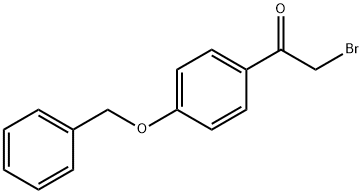
4254-67-5
69 suppliers
$29.00/100mg

56443-72-2
19 suppliers
$125.09/10mg
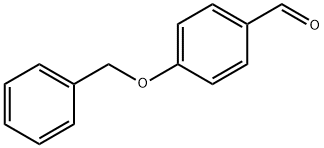
4397-53-9
325 suppliers
$8.00/10g

56443-72-2
19 suppliers
$125.09/10mg