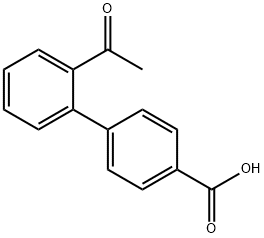
2'-ACETYL[1,1'-BIPHENYL]-4-CARBOXYLIC ACID synthesis
- Product Name:2'-ACETYL[1,1'-BIPHENYL]-4-CARBOXYLIC ACID
- CAS Number:893737-17-2
- Molecular formula:C15H12O3
- Molecular Weight:240.25
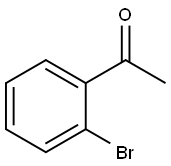
2142-69-0
319 suppliers
$6.00/1g
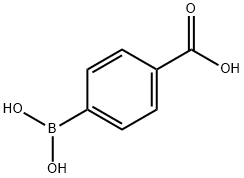
14047-29-1
466 suppliers
$10.00/5G
![2'-ACETYL[1,1'-BIPHENYL]-4-CARBOXYLIC ACID](/StructureFile/ChemBookStructure8/GIF/CB4684413.gif)
893737-17-2
9 suppliers
$70.00/500mg
Yield:893737-17-2 91%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in acetonitrile at 90; for 22 h;
Steps:
51.A
Example 51 10-[(2'-ACETYL-1,1'-BIPHENYL-4-YL)CARBONYL]-N-(PYRIDIN-3-YLMETHYL)-10,11-DIHYDRO-5H-PYRROLO[2,1-C][1,4]BENZODIAZEPINE-3-CARBOXAMIDE Step A. 2'-Acetyl-biphenyl-4-carboxylic acid; To a suspension of 4-carboxybenzeneboronic acid (2.0 g, 12.1 mmol) and 1-(2-bromo-phenyl)-ethanone (1.63 mL, 12.1 mmol) in dry acetonitrile (60 mL) was added a 0.4 M aqueous sodium carbonate solution (60 mL) and the reaction mixture purged with nitrogen for 10 minutes. Tetrakis(triphenylphosphine)palladium(0) (0.5 g, 0.433 mmol) was then added and the reaction mixture heated to 90° C. for 22 hours. The hot reaction mixture was filtered through celite and then concentrated in vacuo to remove acetonitrile. The resulting aqueous suspension was diluted with water to 75 mL then washed with diethyl ether (75 mL). The aqueous phase was acidified to pH 1 by the addition of concentrated hydrochloric acid and the resulting white suspension cooled to 4° C. for 1 hour. The solid product was filtered, washed with water and then dried in vacuo at 50° C. overnight to afford the title compound (2.62 g, 91%) as a white solid, m.p. 199-201° C. MS [(+)ESI, m/z]: 241 [M+H]+
References:
US2006/287522,2006,A1 Location in patent:Page/Page column 38