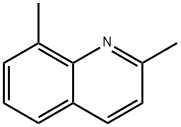
2,8-Dimethylquinoline synthesis
- Product Name:2,8-Dimethylquinoline
- CAS Number:1463-17-8
- Molecular formula:C11H11N
- Molecular Weight:157.21

64-17-5
724 suppliers
$10.00/50g
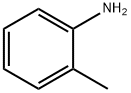
95-53-4
436 suppliers
$10.00/1g

1463-17-8
117 suppliers
$30.00/250mg
Yield:1463-17-8 94%
Reaction Conditions:
Stage #1:ethanol with iron chromite for 24 h;Fenton Reaction;
Stage #2: with dihydrogen peroxide in water at 20; for 2 h;Irradiation;Fenton Reaction;
Stage #3:o-toluidine in water at 20; for 0.0833333 h;
Steps:
Photoactivated tandem synthesis of substituted quinolines 3a-e, 4a-e, and 7a-d (General method).
General procedure: Synthesis was carried out in a Lelesil Innovative Systems photocatalytic reactor with a 500-ml quartz reactor. Fe(CrO2)2 (1.07 g, 4 mol %) and the corresponding alcohol (120 mmol) were placed in the reactor flask, shaken, and left for 24 h. Just before the reaction, the suspension of the catalyst in alcohol was subjected to ultrasonic treatment for 10 min. To the obtained suspension, aq H2O2 (5 wt %, 102 ml,150 mmol) was added. The reactor was connected to the installation in accordance with the manufacturer's instructions. The source of the radiation was a medium-pressure Hg lamp with a power of 250 W. The spectral composition of the radiation was as follows (in terms of energy): UV(48%), visible (43%), and IR (9%). The range of wavelengths was 222-1368 nm. A light flux reached the reaction system passing through an aqueous layer thermostated at 20°C. Photoactivation was performed within 2 h. Then, aniline (1) (4.66 g, 50 mmol) or arylamine 6a-c (50 mmol) was added. The reaction mixture was stirred for 5 min. After completion of the reaction, the mixture was extracted with Et2O (100 ml). The upper organic phase was separated, dried with anhydrous MgSO4, and Et2O was removed under reduced pressure. The residue was fractionally distilled under reduced pressure to afford products 3a-e, 4a-e, and 7a-d.
References:
Makhmutov, Aynur R.;Mustafin, Akhat G.;Usmanov, Salavat M. [Chemistry of Heterocyclic Compounds,2018,vol. 54,# 3,p. 369 - 374][Khim. Geterotsikl. Soedin.,2018,vol. 54,# 3,p. 369 - 374,6]
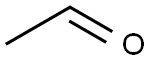
75-07-0
418 suppliers
$14.00/5mL

95-53-4
436 suppliers
$10.00/1g

1463-17-8
117 suppliers
$30.00/250mg
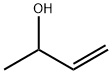
598-32-3
156 suppliers
$15.00/1g

95-53-4
436 suppliers
$10.00/1g

1463-17-8
117 suppliers
$30.00/250mg
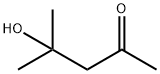
123-42-2
577 suppliers
$19.00/100ml

84902-24-9
26 suppliers
$140.00/250mg

1463-17-8
117 suppliers
$30.00/250mg

871507-79-8
34 suppliers
$83.00/250mg
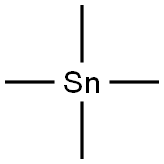
594-27-4
115 suppliers
$28.00/5g
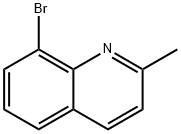
61047-43-6
106 suppliers
$60.00/1 g

1463-17-8
117 suppliers
$30.00/250mg