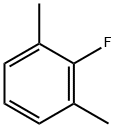
2,6-Dimethylfluorobenzene synthesis
- Product Name:2,6-Dimethylfluorobenzene
- CAS Number:443-88-9
- Molecular formula:C8H9F
- Molecular Weight:124.16
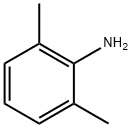
87-62-7
451 suppliers
$10.00/5 g

443-88-9
187 suppliers
$10.00/5g
Yield:443-88-9 86.3%
Reaction Conditions:
with sodium nitrite in Et3N-3HF;diethyl ether
Steps:
2 Diazotization of 2,6-dimethylaniline in Et3N-3HF
EXAMPLE 2 Diazotization of 2,6-dimethylaniline in Et3N-3HF The diazotization of 2,6-dimethylaniline (2.3 g, 0.02 mol) was performed under the same conditions as described for 2,4,6-trimethylaniline in Example 1. The reaction mixture turned yellow during the initial addition of sodium nitrite (2.0 g, 0.03 mol) and gradually turned red with the increased addition of sodium nitrite. Some tar was formed which was easily extracted with solvent. The work-up was identical to that described for 2,4,6-trimethylaniline. Diethyl ether (150 cm3) was used for the extraction of the organic layer from the aqueous washings. The organic extract was dried over magnesium sulphate. Fractional distillation of the solvent formed an orange oil which was distilled at 141-143° C. ? atmospheric pressure affording 1-fluoro-2,6-dimethylbenzene (2.0 g, 86.3%) as a colourless liquid. The distillation was complete in 1 hour. The 1H n.m.r. spectrum contained signals at δH (CDCl3) 2.25, 2-CH3 and 6-CH3 (d, J=2.0 Hz, 6H); 6.91, 3-H and 5-H (dd, J=8.3 Hz and J=6.5 Hz. 2H) and 7.01, 4-H (t, J=8.0 Hz, 1H). The 19F n.m.r. spectrum had a signal at δF (CDCl3) 122.5, 1-F (ts, J=6.5 Hz and J=2.0 Hz). The mass spectrum produced a molecular ion at m/z 124 and the expected fragmentation pattern for 1-fluoro-2,6-dimethylbenzene at m/z 109, 103, 96, 89, 83 and 77.
References:
Rhodia Limited US6179970, 2001, B2
876-99-3
1 suppliers
inquiry

443-88-9
187 suppliers
$10.00/5g
608-28-6
187 suppliers
$9.00/1g

443-88-9
187 suppliers
$10.00/5g
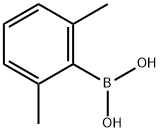
100379-00-8
295 suppliers
$8.00/1g

443-88-9
187 suppliers
$10.00/5g
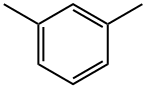
108-38-3
363 suppliers
$10.60/50gm:

443-88-9
187 suppliers
$10.00/5g
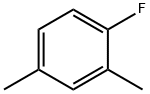
452-65-3
53 suppliers
$17.00/250mg