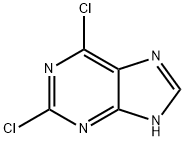
2,6-Dichloropurine synthesis
- Product Name:2,6-Dichloropurine
- CAS Number:5451-40-1
- Molecular formula:C5H2Cl2N4
- Molecular Weight:189
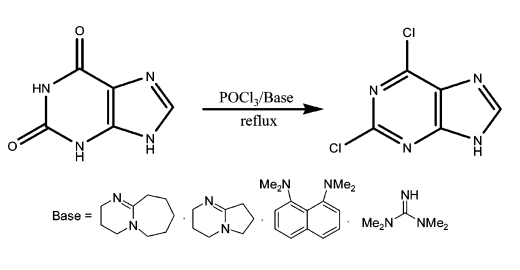
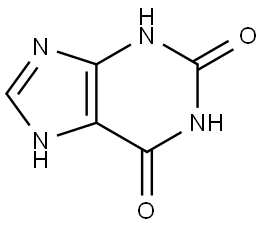
(1) By chlorination of the purine ring structure, e.g., chlorination of xanthine (2,6-dihydroxypurine) with pyrophosphoryl chloride at high temperatures in sealed tubes in the presence of a phase-transfer catalyst or with phosphorus oxychloride under reflux, and chlorination of 6-chloropurine, hypoxanthine, or their N-oxides with phosphorus oxychloride, and chlorination with chlorine gas at low temperatures. Chlorination of 2,6-dithiopurine.
(2) The purine ring is constructed using barbituric acid derivatives or 2,4-dichloro-5,6-diaminopyridine as starting materials. However, both methods are not very suitable for industrial production.
The industrial preparation of 2,6-dichloropurine involves the direct chlorination of xanthine with phosphorus trichloride and a weakly nucleophilic organic base (e.g., amidine, guanidine base, or proton sponge)[1]. The reaction process is shown below:
10310-21-1
555 suppliers
$10.00/5g

5451-40-1
501 suppliers
$10.00/1g
Yield:5451-40-1 99%
Reaction Conditions:
with hydrogenchloride;1,3-dimethylimidazolium chloride;NaNO2 in lithium hydroxide monohydrate at 10; for 2 h;Reagent/catalyst;Temperature;
Steps:
1 Example 3
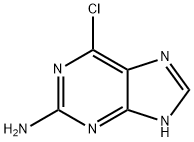
1) In a 2L reaction kettle, first put 750 g of ionic liquid 1,3-dimethylimidazolium chloride [DMIm]Cl, and stir. Next, 150 g of concentrated hydrochloric acid was added. Continue to add 100 g of 2-amino-6-chloropurine under stirring.2) Continue stirring, control the temperature at 10°C, and slowly add 55 g of sodium nitrite to carry out the reaction. The reaction time was 2 hours.3) After the reaction, extract with ethyl acetate (500ml*4) to obtain the crude product, concentrate and recrystallize from methanol to obtain 110g of 2,6-dichloropurine white crystals. Melting point: 180182°C, yield 99.0%, content 99.5%.4) The mother liquor after ethyl acetate extraction was fully cooled at -10°C for 24 hours to separate out the material, and the filtrate obtained after filtering and removing impurities was directly applied to the next batch of reactions. After the concentrated hydrochloric acid is supplemented, the reaction can be carried out stably, the yield of the final product is 98.2%, and the content is 99.2%.
References:
CN114349754,2022,A Location in patent:Paragraph 0024-0047
69-89-6
483 suppliers
$6.00/1g

5451-40-1
501 suppliers
$10.00/1g

10310-21-1
555 suppliers
$10.00/5g
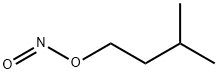
110-46-3
280 suppliers
$10.00/10g

5451-40-1
501 suppliers
$10.00/1g

10310-21-1
555 suppliers
$10.00/5g

5451-40-1
501 suppliers
$10.00/1g

69-89-6
483 suppliers
$6.00/1g

5451-40-1
501 suppliers
$10.00/1g