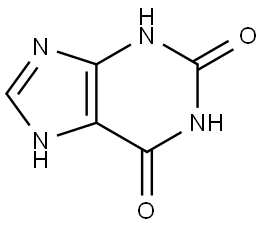
Xanthine synthesis
- Product Name:Xanthine
- CAS Number:69-89-6
- Molecular formula:C5H4N4O2
- Molecular Weight:152.11
Yield:69-89-6 9.2 g (60%)
Reaction Conditions:
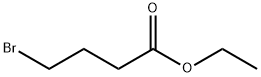
in sodium hydroxide;(CH2)3 COOEt;N,N-dimethyl-formamide;
Steps:
2 (Method B, xanthine no. 21)
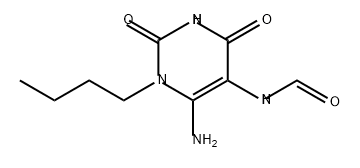
EXAMPLE 2 1-(4-hydroxybutyl)-3-butyl xanthine (Method B, xanthine no. 21) 11.3 g (0. 05 mole) of 1-butyl-5-formylamino-6-aminouracil (II) (R1 =butyl, R8 =H) are dissolved under nitrogen in 200 ml of DMF. 10.6 ml (0.075 mole) of ethyl 4-bromobutyrate are added, after which 2 g (0.05 mole) of solid powder-form NaOH is added with thorough stirring in portions of 0.5 g at intervals of 1 hour. On completion of the addition, the mixture is left to react overnight. The solvent is then evaporated and the oily residue of (IV) (substituent in the 1-position=(CH2)3 COOEt) is dissolved in 100 ml of 10% NaOH. This solution is then heated under reflux for 0.5 hour and then cooled, neutralized to pH 5 with acetic acid and filtered. The precipitate formed is dried. Yield: 9.2 g (60%) of crude xanthine (V) (substituent in the 1-position=(CH2)3 COOH, R3 =butyl, R8 =H).
References:
US5338741,1994,A
73-40-5
697 suppliers
$5.00/5g

69-89-6
484 suppliers
$6.00/1g
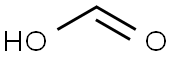
64-18-6
1084 suppliers
$22.12/250ML
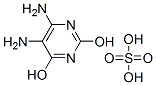
42965-55-9
24 suppliers
$122.00/5g

69-89-6
484 suppliers
$6.00/1g
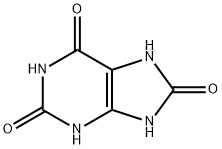
69-93-2
237 suppliers
$30.00/20mg

69-89-6
484 suppliers
$6.00/1g
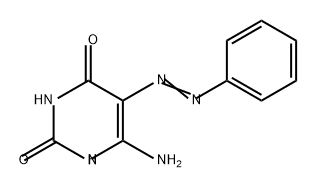
6979-73-3
0 suppliers
inquiry

69-89-6
484 suppliers
$6.00/1g