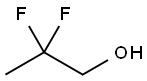
2,2-Difluoropropanol synthesis
- Product Name:2,2-Difluoropropanol
- CAS Number:33420-52-9
- Molecular formula:C3H6F2O
- Molecular Weight:96.08
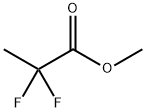
38650-84-9
41 suppliers
$45.00/50mg

33420-52-9
185 suppliers
$21.00/1g
Yield:33420-52-9 65%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20;Inert atmosphere;Cooling with ice;
Steps:
2 [Reference Example 2]
Under a nitrogen atmosphere, to a tetrahydrofuran 1.80L (0.870L / mol),lithium aluminum hydride 45.7g (1.20mol, 0.580eq) was added and stirred underice-cooling. In this heterogeneous solution, the following formula ,In α shown, α- difluoro esters 257g (2.07mol, 1.00eq) tetrahydrofuransolution of the (solvent usage 300mL) was added under ice-cooling, and themixture was stirred overnight at room temperature. The conversion rate of F-NMR analysis of the reaction solution was 100%. To the end of thereaction solution, water 220g (12.2mol, 5.89eq) tetrahydrofuran solution of the(solvent usage 220mL) was added under ice-cooling, the temperature wasgradually raised to 60 ° C, and the mixture was stirred for 3 hours at the sametemperature ( suspended solution). The suspension solution was filtered throughCelite and washed with tetrahydrofuran 300mL, the following formula In indicated β,obtained 2.40kg a tetrahydrofuran solution containing β- difluoro alcohol.Internal standard method of F-NMR of the tetrahydrofuran solution(internal standard alpha, alpha, α-trifluoro toluene) was quantified inthe desired compound were included 1.66 mol. In addition, the moisture contentthan Karl Fischer analysis of the tetrahydrofuran solution was 5.1%.In tetrahydrofuransolution obtained in the above, in addition to tetrahydrofuran 630mL, performazeotropic dehydration by atmospheric distillation (distillation temperature~66 ° C), followed recovery distillation (degree of vacuum ~16kPa, distillationtemperature to 64 the ° C) to, beta represented by the above formula, the crudeproduct of β- difluoro alcohol (containing a substantial amount of tetrahydrofuran)was obtained 693 g. Of crude product F-NMR of the internal standardmethod (internal standard alpha, alpha, α-trifluoro toluene) wasquantified by, contains the desired compound 1.59 mol, yield 77 %Met. Inaddition, the moisture content than Karl Fischer analysis of the crude productwas 1.6%.
References:
CENTRAL GLASS COMPANY LIMITED;ISHII, AKIHIRO;YAMAZAKI, TAKAKO;TSURUTA, HIDEYUKI;ISHIMARU, TAKEHISA;NISHIMIYA, TAKAYUKI JP5659658, 2015, B2 Location in patent:Paragraph 0154-0158
784193-14-2
8 suppliers
inquiry

33420-52-9
185 suppliers
$21.00/1g
97006-65-0
0 suppliers
inquiry

33420-52-9
185 suppliers
$21.00/1g
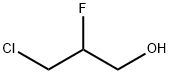
26438-85-7
1 suppliers
inquiry

33420-52-9
185 suppliers
$21.00/1g