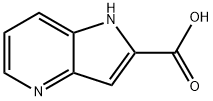
1H-PYRROLO[3,2-B]PYRIDINE-2-CARBOXYLIC ACID synthesis
- Product Name:1H-PYRROLO[3,2-B]PYRIDINE-2-CARBOXYLIC ACID
- CAS Number:17288-35-6
- Molecular formula:C8H6N2O2
- Molecular Weight:162.15
![1H-Pyrrolo[3,2-b]pyridine-2-carboxylic acid ethyl ester](/CAS2/GIF/17288-32-3.gif)
17288-32-3
119 suppliers
$26.00/250mg
![1H-PYRROLO[3,2-B]PYRIDINE-2-CARBOXYLIC ACID](/CAS/GIF/17288-35-6.gif)
17288-35-6
98 suppliers
$21.00/100mg
Yield: 82%
Reaction Conditions:
Stage #1:1H-pyrrolo[3,2-b]pyridine-2-carboxylic acid ethyl ester with lithium hydroxide in ethanol;water for 16 h;
Stage #2: in ethanol;water; pH=4Acidic conditions;
Steps:
4.2.2.1. 1H-Pyrrolo[3,2-b]pyridine-2-carboxylic acid (5f)
3-Amino-2-chloro-pyridine 4f (150 mg, 1.17 mmol), ethyl pyruvate 8 (0.25 ml, 2.00 mmol), pyridinium p-toluenesulfonate, (73 mg, 0.29 mmol) and tetraethoxy-silane (0.26 ml, 1.18 mmol) were suspended in 0.4 ml pyridine and stirred for 24 h at 20 °C. Afterwards Pd[P(C6H6)3]4 (70 mg, 0.06 mmol) and N,N-dicyclohexylmethylamine (0.35 ml, 2.06 mmol) were added and the reaction mixture was heated in a microwave oven to 160 °C for 20 min. The reaction mixture is diluted with 100 ml dichloromethan and extracted two times with 50 ml of a half saturated aqueous sodium hydrogencarbonat solution. The organic layer was dried with sodium sulfate, the solvent was evaporated under reduced pressure and the crude product was purified using chromatography method P3, yielding 190 mg (1.00 mmol) of 1H-Pyrrolo[3,2-b]pyridine-2-carboxylic acid ethyl ester. The ester was dissolved in 17 ml ethanol and 5 ml water. To this solution lithium hydroxide (120 mg, 5.00 mmol) was added. After 16 h the pH value of the reaction mixture was adjusted to pH 4 and the solvent is evaporated in vacuum. The crude product was purified using an acid ion exchanger (Strata-X-C, Phenomenex), yielding of 155 mg (82%) of the title compound. Purity by method A1: >95%; MS (ESI) m/z 163 (M + H)+; 1H NMR (DMSO) δ (ppm) 13.34 (br, 1H), 8.77 (d, J = 5.3 Hz, 1H), 8.53 (d, J = 8.3 Hz, 1H), 7.73 (dd, J = 5.4 Hz, J = 8.3 Hz, 1H), 7.33 (br, 1H); 13C NMR (500 MHz, DMSO) δ (ppm) 161.4 (s), 138.0 (s), 136.1 (s), 135.8 (s), 132.7 (s), 128.6 (s), 119.6 (s), 101.2 (s).
References:
Engelhardt, Harald;De Esch, Iwan J.P.;Kuhn, Daniel;Smits, Rogier A.;Zuiderveld, Obbe P.;Dobler, Julia;Mayer, Moriz;Lips, Sebastian;Arnhof, Heribert;Scharn, Dirk;Haaksma, Eric E.J.;Leurs, Rob [European Journal of Medicinal Chemistry,2012,vol. 54,p. 660 - 668] Location in patent:experimental part
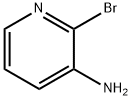
39856-58-1
304 suppliers
$10.00/1g
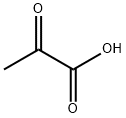
127-17-3
529 suppliers
$5.00/10g
![1H-PYRROLO[3,2-B]PYRIDINE-2-CARBOXYLIC ACID](/CAS/GIF/17288-35-6.gif)
17288-35-6
98 suppliers
$21.00/100mg
![1H-Pyrrolo[3,2-b]pyridine-2-carboxylic acid ethyl ester](/CAS2/GIF/17288-32-3.gif)
17288-32-3
119 suppliers
$26.00/250mg
![1h-pyrrolo[3,2-b]pyridine-2-carboxaMide](/CAS/20150408/GIF/853685-35-5.gif)
853685-35-5
6 suppliers
inquiry
![1H-PYRROLO[3,2-B]PYRIDINE-2-CARBOXYLIC ACID](/CAS/GIF/17288-35-6.gif)
17288-35-6
98 suppliers
$21.00/100mg
18699-87-1
248 suppliers
$8.00/1g
![1H-PYRROLO[3,2-B]PYRIDINE-2-CARBOXYLIC ACID](/CAS/GIF/17288-35-6.gif)
17288-35-6
98 suppliers
$21.00/100mg
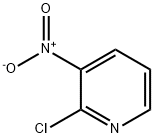
5470-18-8
439 suppliers
$6.00/5g
![1H-PYRROLO[3,2-B]PYRIDINE-2-CARBOXYLIC ACID](/CAS/GIF/17288-35-6.gif)
17288-35-6
98 suppliers
$21.00/100mg