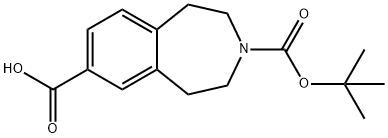
3-(TERT-BUTOXYCARBONYL)-2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPINE-7-CARBOXYLIC ACID synthesis
- Product Name:3-(TERT-BUTOXYCARBONYL)-2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPINE-7-CARBOXYLIC ACID
- CAS Number:149353-73-1
- Molecular formula:C16H21NO4
- Molecular Weight:291.34
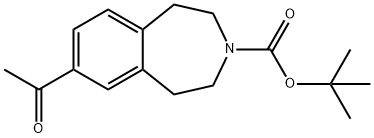
264264-31-5
15 suppliers
$1408.00/500mg
![3-(TERT-BUTOXYCARBONYL)-2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPINE-7-CARBOXYLIC ACID](/CAS/GIF/149353-73-1.gif)
149353-73-1
29 suppliers
inquiry
Yield:149353-73-1 100%
Reaction Conditions:
with water;bromine;sodium hydroxide in 1,4-dioxane at 0;
Steps:
1.1.4
1.1.4 Intermediate 4; To a solution of tert-huty 7-acetyl-4,5-dihydro-lH-benzo[d]azepine-3(2H)-carboxylate (3) (2g, 6.9mmol) in dioxane (23ml) was added an aqueous solution of sodium hydroxide (2.2g, 55.2mmol in 32ml of water) drop wise. The reaction mixture was then cooled to 0° C and bromine (1.06ml, 20.7mmol) added drop wise. The reaction was allowed to stir at 0° C for 1 hour. The reaction was carefully quenched with acetone and the reaction reduced in vacuo. The remaining aqueous layer was washed with ethyl acetate and then acidified with 5N hydrochloride acid. The aqueous layer was then extracted with ethyl acetate and the combined organics washed with brine and dried over magnesium sulphate to give 3(tert-butoxycarbonyl)-2,3,4,5-tetrahydro-lH- benzo[d]azepi?e-7-carboxylic acid (4) as a pale yellow solid in quantitative yield.MS ES+ : 192IH NMR (400 MHz, DMSO-d6) δ 7.60 - 7.80 (m, 2H), 7.16 - 7.32 (m, IH), 3.41 - 3.52 (m, 4H), 2.84 - 2.97 (m, 4H), 1.34 - 1.47 (m, 9H)
References:
WO2010/7382,2010,A1 Location in patent:Page/Page column 21; 23
871501-42-7
0 suppliers
inquiry

24424-99-5
833 suppliers
$13.50/25G
![3-(TERT-BUTOXYCARBONYL)-2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPINE-7-CARBOXYLIC ACID](/CAS/GIF/149353-73-1.gif)
149353-73-1
29 suppliers
inquiry
![2,3,4,5-Tetrahydro-1H-benzo[d]azepine-7-carboxylic acid](/CAS/GIF/175714-52-0.gif)
175714-52-0
3 suppliers
inquiry

24424-99-5
833 suppliers
$13.50/25G
![3-(TERT-BUTOXYCARBONYL)-2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPINE-7-CARBOXYLIC ACID](/CAS/GIF/149353-73-1.gif)
149353-73-1
29 suppliers
inquiry
7646-93-7
227 suppliers
$10.00/10g
![2,3,4,5-Tetrahydro-1H-benzo[d]azepine-7-carboxylic acid](/CAS/GIF/175714-52-0.gif)
175714-52-0
3 suppliers
inquiry

24424-99-5
833 suppliers
$13.50/25G
![3-(TERT-BUTOXYCARBONYL)-2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPINE-7-CARBOXYLIC ACID](/CAS/GIF/149353-73-1.gif)
149353-73-1
29 suppliers
inquiry
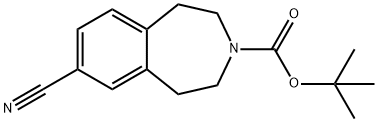
149354-02-9
2 suppliers
inquiry
![3-(TERT-BUTOXYCARBONYL)-2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPINE-7-CARBOXYLIC ACID](/CAS/GIF/149353-73-1.gif)
149353-73-1
29 suppliers
inquiry