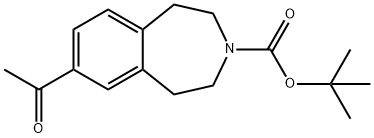
tert-Butyl7-acetyl-2,3,4,5-tetrahydro-1H-3-benzazepine-3-carboxylate synthesis
- Product Name:tert-Butyl7-acetyl-2,3,4,5-tetrahydro-1H-3-benzazepine-3-carboxylate
- CAS Number:264264-31-5
- Molecular formula:C17H23NO3
- Molecular Weight:289.37
![1-(2,3,4,5-tetrahydro-1H-benzo[d]azepin-7-yl)ethanone](/CAS/20150408/GIF/34685-20-6.gif)
34685-20-6
5 suppliers
inquiry

24424-99-5
861 suppliers
$13.50/25G

264264-31-5
14 suppliers
$1408.00/500mg
Yield:-
Reaction Conditions:
with potassium carbonate in 1,4-dioxane;water at 20; for 48 h;
Steps:
1.1.3
1.1.3 Intermediate 3; To a suspension of l-(7-acetyl-4,5-dihydro-lH-benzo[d]azepin-3(2H)-yl)-2,2,2-trifluoroethanone (2) (1Og, 35mmol) in methanol (496ml) was added water (164ml) and saturated potassium carbonate solution (164ml) and the reaction allowed to stir at room temperature for 16 hours. The reaction mixture was concentrated in vacuo and the residue partitioned between ethyl acetate and water. The aqueous layer was washed with ethyl acetate and the combined organics washed with brine and dried over magnesium sulphate. The residue was azeotroped with toluene and the material used directly.MS ES+ : 190IH NMR (400 MHz, DMSO-d6): δ 7.64 - 7.70 (m, 2H), 7.20 - 7.26 (m, IH), 2.86 - 2.93 (m, 4H), 2.73 - 2.80 (m, 4H), 2.53 (s, 3H)).To a solution of l-(2,3,4,5-tetrahydro-lH-benzo[d]azepin-7-yl)ethanone (10.44g, 35mmol) in dioxane (93ml) and water (35ml) was added Boc anhydride (7.64g, 35mmol) and potassium carbonate (4.84g, 35mmol) and the reaction allowed to stir at room temperature for 48 hours. The reaction mixture was concentrated in vacuo and partitioned between ethyl acetate and aqueous saturated sodium hydrogen carbonate solution. The combined organics were washed with brine and dried over magnesium sulphate. The crude oil was crystallised over night to give tert-buty 7-acetyl-4,5-dihydro-lH-benzo[d]azepine-3(2H)-carboxylate (3) as a cream solid in 16.8mmol.MS ES+ : 189, ES: 216IH NMR (400 MHz, DMSO-d6) δ 7.66 - 7.74 (m, 2H), 7.21 - 7.30 (m, IH), 3.37 - 3.48 (m, 4H), 2.83 - 2.93 (m, 4H), 2.50 (s, 3H), 1.36 (s, 9H)
References:
WO2010/7382,2010,A1 Location in patent:Page/Page column 21-23