
6-chloro-5-(4-(1-hydroxycyclobutyl)phenyl)-1H-indole-3-carboxylicacid synthesis
- Product Name:6-chloro-5-(4-(1-hydroxycyclobutyl)phenyl)-1H-indole-3-carboxylicacid
- CAS Number:1467057-23-3
- Molecular formula:C19H16ClNO3
- Molecular Weight:341.79
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1467057-23-3
63 suppliers
$25.00/1mg
Yield: 71%
Reaction Conditions:
with sodium chlorite;sodium dihydrogen phosphate monohydrate;2-methyl-but-2-ene in water;acetonitrile;tert-butyl alcohol at 20; for 22 h;
Steps:
1.4 6-Chloro-5-(4-(1-hydroxycyclobutyl)phenyl)-1H-indole-3-carboxylic acid
To a solution of 6-chloro-5-[4-(1-hydroxy-cyclobutyl)-phenyl]-1H-indole-3-carbaldehyde (4.88 g, 15.0 mmol) in MeCN (212 mL) and tert-butanol (212 mL) at 0° C. was added 2-methyl-2-butene (120 mL, 1.15 mol), followed by a solution of sodium chlorite (25.5 g, 300 mmol) and sodium phosphate monobasic hydrate (42.5 g, 308 mmol) in water (212 mL) dropwise via addition funnel.
The ice bath was removed and the reaction mixture was stirred vigorously at room temperature.
After 13 hours, additional 2-methyl-2-butene (50 mL, 480 mmol) was added, followed by sodium chlorite (10.6 g, 125 mmol) and sodium phosphate monobasic hydrate (17.7 g, 125 mmol) as solids.
The reaction mixture was stirred at room temperature for an additional 5 hours, and treated with additional 2-methyl-2-butene (25 mL, 240 mmol), solid sodium chlorite (5.3 g, 73 mmol) and solid sodium phosphate monobasic hydrate (8.8 g, 73 mmol).
After an additional 4 hours, the reaction mixture was poured into a 4:1 mixture of saturated aqueous NH4Cl solution/water (500 mL), and extracted with EtOAc (3*400 mL).
The combined organic layers were dried over Na2SO4 and concentrated in vacuo.
The resulting material was loaded onto a silica gel plug and eluted, first with heptane/EtOAc (4:1, 1.5 L), followed by 1:4 heptane/EtOAc (3 L) then EtOAc (1 L).
The filtrates from the second and third elutions were combined and concentrated in vacuo.
The resulting tan solid was partially dissolved in 4:1 DMF/DCM, loaded onto an Isco silica gel cartridge, and purified by flash chromatography (20-80% EtOAc/heptane, with 0.2% formic acid modifier) to give the title compound (3.65 g, 71%) as a white solid. MS (ES+) 340.2 (M-H)+. 1H NMR (500 MHz, DMSO-d6) δ 12.10 (s, 1H), 11.95 (s, 1H), 8.08 (s, 1H), 7.95 (s, 1H), 7.64 (s, 1H), 7.57 (d, J=7.1 Hz, 2H), 7.41 (d, J=7.1 Hz, 2H), 5.52 (s, 1H), 2.48-2.40 (m, 2H), 2.35-2.26 (m, 2H), 1.97-1.90 (m, 1H), 1.76-1.62 (m, 1H).
References:
PFIZER INC.;Bhattacharya, Samit;Cameron, Kimberly;Dowling, Matthew;Fernando, Dilinie;Ebner, David;Filipski, Kevin;Kung, Daniel;Lee, Esther;Smith, Aaron;Tu, Meihua US2013/267493, 2013, A1 Location in patent:Paragraph 0696; 0700
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1467057-23-3
63 suppliers
$25.00/1mg
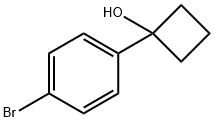
19936-14-2
70 suppliers
$43.00/100mg

1467057-23-3
63 suppliers
$25.00/1mg
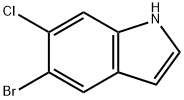
122531-09-3
128 suppliers
$7.00/100mg

1467057-23-3
63 suppliers
$25.00/1mg
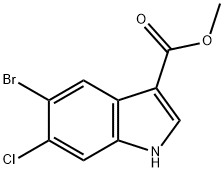
1467059-91-1
34 suppliers
$505.85/5MG
![[4-(1-hydroxycyclobutyl)phenyl]boronic acid](/CAS/20200331/GIF/1467062-11-8.gif)
1467062-11-8
10 suppliers
inquiry

1467057-23-3
63 suppliers
$25.00/1mg