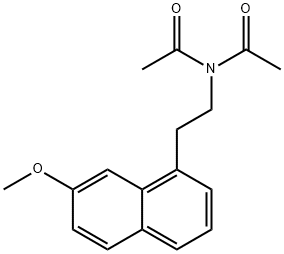
N-acetyl-N-(2-(7-Methoxynaphthalen-1-yl)ethyl)acetaMide synthesis
- Product Name:N-acetyl-N-(2-(7-Methoxynaphthalen-1-yl)ethyl)acetaMide
- CAS Number:1379005-34-1
- Molecular formula:C17H19NO3
- Molecular Weight:285.34
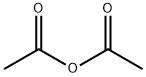
108-24-7
0 suppliers
$14.00/250ML
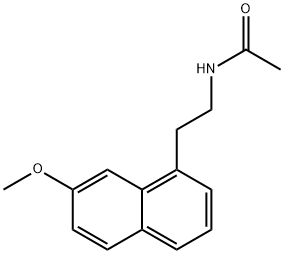
138112-76-2
597 suppliers
$5.00/5mg

1379005-34-1
40 suppliers
inquiry
Yield:1379005-34-1 31%
Reaction Conditions:
Stage #1: agomelatinewith hydrogenchloride in water; pH=Ca. 1.5;
Stage #2: acetic anhydridewith sodium hydrogencarbonate in water; pH=Ca.5.5;Cooling with ice;
Steps:
1 Example 1: Formula IIA
Formula IIA: To a heterogeneous suspension of N-[2-(7-methoxy-l-naphthyl)ethyl]- acetamide (1 mmol) in water (5 mL), 6N HC1 (in the volume range of 240-400 μ?) is added until the solution becomes homogeneous (pH ca.1.5). The resulting homogenous solution is cooled in an ice bath. Acetic anhydride (-1-1.5 mmol) is added, followed by addition of solid sodium bicarbonate (-185-300 mg) until there is no further effervescence, or the pH of the reaction mixture becomes approximately about 5.5. The precipitated product is filtered, washed with water (2 x 1 mL), and dried by pressing between folds of filter paper. Traces of water are removed in a vacuum desiccator to obtain N-acetyl-N-(2-(7-methoxynaphthalen-l-yl)ethyl)- acetamide, II-A. The yield was 31%. Molecular Formula = C18H19NO3 Formula Weight = 297.34836 and CHN Composition = C(72.71%) H(6.44%) N(4.71%) 0(16.14%). [0048] 1H NMR (300 MHz, DMSO-d6) d 1.83 (s, 3H), 3.12 (t, J = 8.3 Hz, 2H), 3.33, (m, 2H), 3.94 (s, 3H), 3.88 (s, 3H), 7.20 (dd, J = 9.0 and 2.4 Hz, 1H), 7.33 (d, J = 2.0 Hz, 1H), 7.45 (d, J = 2.4 Hz, 1H), 7.55 (d, J = 2.4 Hz, 1H) and 7.68 (d, J = 9.0 Hz, 1H). Mass Spectra- M+ = 297.135945 Da, M- = 297.137042 Da, [M+H] + = 298.14377 Da, [M+H]- = 298.144867 Da, [M- H]+ =296.12812 Da and [M-H]- = 296.129217 Da.
References:
WO2013/13060,2013,A1 Location in patent:Paragraph 0047-0048