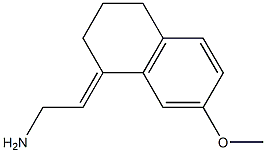
(E)-2-(7-methoxy-3,4-dihydronaphthalen-1(2H)-ylidene)ethan-1-amine synthesis
- Product Name:(E)-2-(7-methoxy-3,4-dihydronaphthalen-1(2H)-ylidene)ethan-1-amine
- CAS Number:1371535-54-4
- Molecular formula:C13H17NO
- Molecular Weight:203.2802
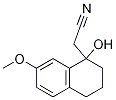
59081-65-1
4 suppliers
inquiry

1371535-54-4
16 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 2-(1-hydroxyl-7-methoxy-1,2,3,4-tetrahydro-naphthalen-1-yl)-acetonitrilewith ammonia;hydrogen;Raney-Ni in methanol;water at 25 - 30; for 5 h;autoclave;
Stage #2: with hydrogenchloride in ethyl acetate at 25 - 35; pH=< 2; for 3 h;
Steps:
1; 7
2-( 1 -hydroxy-7-methoxy- 1 ,2,3 ,4-tetrahydronaphthalen- 1 -yl)acetonitrilecompound of formula-4 (100 g) was added to a mixture of Raney-Ni (30 g), methanolic ammonia (600 ml) and water (25 ml) at 25-30°C in autoclave. Hydrogen gas (3-4 kg) was bubbled to the reaction mixture. The reaction mixture was heated to 25-30°C and then stirred for 5 v vhours at same temperature. After completion of the reaction, filtered the reaction mixture at 25-30°C and washed with methanol. Distilled off the solvent completely from the filtrate obtained and co-distilled with methanol. Ethylacetate (600 ml) was added to the obtained residue at 25-30°C and then cooled to same temperature. The pH of the reaction mixture was adjusted to below 2.0 with ethylacetate-hydtochloride and stirred for 3 hours at 25-35°C. The obtained solid was filtered, washed with ethylacetate and then dried to get the title compound. Yield: 103 grams; MP: 136.87°C; Purity (by HPLC): 99.57 %.
References:
WO2012/46253,2012,A2 Location in patent:Page/Page column 26; 30