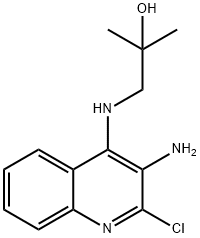
2-Propanol, 1-[(3-amino-2-chloro-4-quinolinyl)amino]-2-methyl- synthesis
- Product Name:2-Propanol, 1-[(3-amino-2-chloro-4-quinolinyl)amino]-2-methyl-
- CAS Number:133860-78-3
- Molecular formula:C13H16ClN3O
- Molecular Weight:265.74
![1-[(2-chloro-3-nitro-4-quinolinyl)amino]-2-methyl-2-propanol](/CAS/20200331/GIF/133860-77-2.gif)
133860-77-2
0 suppliers
inquiry
![2-Propanol, 1-[(3-amino-2-chloro-4-quinolinyl)amino]-2-methyl-](/CAS/20180529/GIF/133860-78-3.gif)
133860-78-3
3 suppliers
inquiry
Yield:133860-78-3 71.5%
Reaction Conditions:
with sodium dithionite;water;triethylamine in isopropyl alcohol at 0; for 1.16667 h;
Steps:
11
To a solution of l-(2-chloro-3-nitroquinolin-4-ylamino)-2-methylpropan-2-ol (5.Og,16.9 mmol) in iPrOH (30 mL) was added triethylamine (17 mL, 12.3g, 122 mmol) followed by water (40 mL). The reaction mixture was cooled to 0 0C and then a solution OfNa2S2O4 (19.5g, 111.9 mmol) in water (80 mL) was added dropwise via dropping funnel over 40 minutes while retaining cooling at 0 0C. Reaction mixture was then stirred at 0 0C for 30 minutes. Cone. HCl (20 mL) was then added and the resulting mixture transferred to a separatory funnel and washed with ethyl acetate (200 mL). The ethyl acetate layer was then extracted with 3 M HCl (50 mL). The combined aqueous extracts were then taken to pH ~7 with addition of KsPO4 (~41 g). The resulting mixture was then extracted with diethyl ether (2x300 mL). The combined ether extracts were washed once with brine, dried over MgSO4, filtered and concentrated. Purification by flash chromatography (silica gel, ethyl
References:
WO2007/109810,2007,A2 Location in patent:Page/Page column 116-117
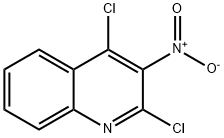
132521-66-5
97 suppliers
inquiry
![2-Propanol, 1-[(3-amino-2-chloro-4-quinolinyl)amino]-2-methyl-](/CAS/20180529/GIF/133860-78-3.gif)
133860-78-3
3 suppliers
inquiry
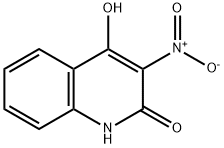
15151-57-2
80 suppliers
inquiry
![2-Propanol, 1-[(3-amino-2-chloro-4-quinolinyl)amino]-2-methyl-](/CAS/20180529/GIF/133860-78-3.gif)
133860-78-3
3 suppliers
inquiry

15151-57-2
80 suppliers
inquiry
![2-Propanol, 1-[(3-amino-2-chloro-4-quinolinyl)amino]-2-methyl-](/CAS/20180529/GIF/133860-78-3.gif)
133860-78-3
3 suppliers
inquiry
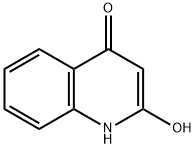
70254-44-3
27 suppliers
inquiry
![2-Propanol, 1-[(3-amino-2-chloro-4-quinolinyl)amino]-2-methyl-](/CAS/20180529/GIF/133860-78-3.gif)
133860-78-3
3 suppliers
inquiry