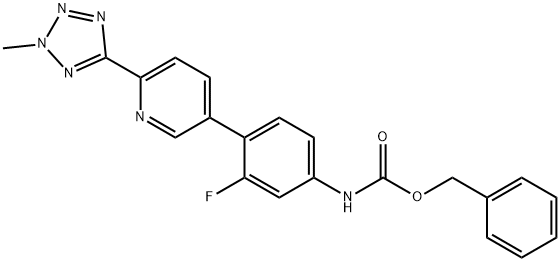
CarbaMic acid, N-[3-fluoro-4-[6-(2-Methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-, phenylMethyl ester synthesis
- Product Name:CarbaMic acid, N-[3-fluoro-4-[6-(2-Methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-, phenylMethyl ester
- CAS Number:1220910-89-3
- Molecular formula:C21H17FN6O2
- Molecular Weight:404.4
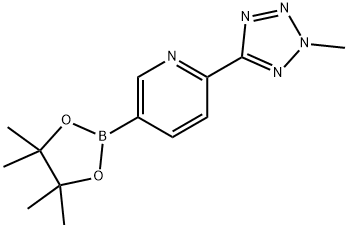
1056039-83-8
120 suppliers
$16.00/250mg
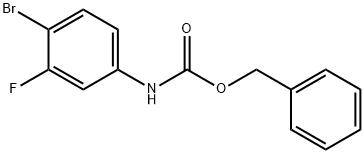
510729-01-8
152 suppliers
$7.00/100mg
![CarbaMic acid, N-[3-fluoro-4-[6-(2-Methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-, phenylMethyl ester](/CAS/GIF/1220910-89-3.gif)
1220910-89-3
132 suppliers
inquiry
Yield: 88%
Reaction Conditions:
with potassium carbonate in water at 80; for 12 h;Inert atmosphere;
Steps:
2 Example 2:synthesis of 3-fluoro-4 - (6 - (2-methyl -2H-tetrazole-5-yl) pyridin-3-yl) phenyl-carbamic acid benzyl ester (compound 1)
Compound 2 (120g, 0.5mol), potassium acetate (145g, 0.5mol) and frequencymellow joint boron is (150g, 0.6mol) 10L in four-mouth bottle, by adding 1,4-dioxane (3L) and Pd (dppf)2Cl2·CH2Cl2(20g, 25mmol). After the protection of nitrogen, heating to 80 °C, reaction 3 hours. Combined liquid detection for determining compound 2 is fully converted to compound 3 the rear, the compound is added 4 (146g, 0.45mol), potassium carbonate (173g, 1.2mol) and water (1L), re-protection of nitrogen, heating to 80 °C reaction 12 hours. LCMS determining the reaction is complete. Filtering, filtering the solid material, the filtrate obtained by reducing pressure and evaporating the evaporimeter 1,4-dioxane, is added to the aqueous phase 500 ml ethanol, beating sleepovers. Filtering, the filter cake is washed with cold-b activity, reduced-pressure drying 4 hours. The crude product in ethyl acetate, heating reflow, holding 5 minutes, the heat filters. Then reducing the temperature to room temperature, the beating sleepovers, filtering, washing the filter cake by ethyl acetate, to obtain compound 1 (177g, 88%), yellow solid.
References:
Cai Ping;Cai, Ping;Chen, Jing CN105367547, 2016, A Location in patent:Paragraph 0034; 0035; 0036
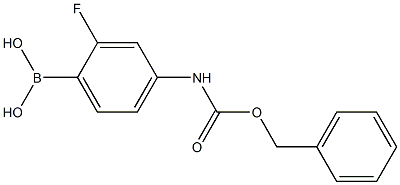
874290-59-2
84 suppliers
$90.00/250mg

380380-64-3
254 suppliers
$10.00/250mg
![CarbaMic acid, N-[3-fluoro-4-[6-(2-Methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-, phenylMethyl ester](/CAS/GIF/1220910-89-3.gif)
1220910-89-3
132 suppliers
inquiry

510729-01-8
152 suppliers
$7.00/100mg
![CarbaMic acid, N-[3-fluoro-4-[6-(2-Methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-, phenylMethyl ester](/CAS/GIF/1220910-89-3.gif)
1220910-89-3
132 suppliers
inquiry

380380-64-3
254 suppliers
$10.00/250mg
![CarbaMic acid, N-[3-fluoro-4-[6-(2-Methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-, phenylMethyl ester](/CAS/GIF/1220910-89-3.gif)
1220910-89-3
132 suppliers
inquiry
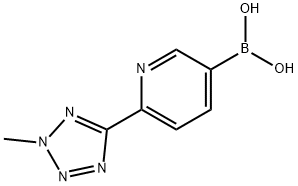
883231-14-9
14 suppliers
inquiry
![CarbaMic acid, N-[3-fluoro-4-[6-(2-Methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-, phenylMethyl ester](/CAS/GIF/1220910-89-3.gif)
1220910-89-3
132 suppliers
inquiry