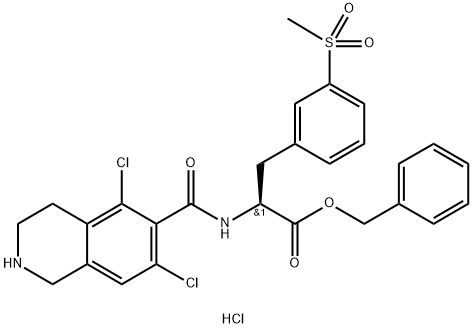
L-Phenylalanine, N-[(5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester, hydrochloride (1:1) synthesis
- Product Name:L-Phenylalanine, N-[(5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester, hydrochloride (1:1)
- CAS Number:1194550-65-6
- Molecular formula:C27H27Cl3N2O5S
- Molecular Weight:597.93
851784-82-2
172 suppliers
$11.00/250mg
1194550-59-8
130 suppliers
$43.00/1g
![L-Phenylalanine, N-[(5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester, hydrochloride (1:1)](/CAS/20211123/GIF/1194550-65-6.gif)
1194550-65-6
55 suppliers
inquiry
Yield:1194550-65-6 98%
Reaction Conditions:
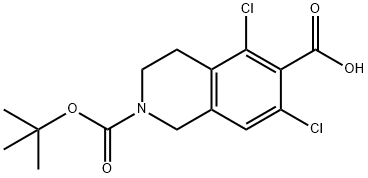
Stage #1:2-(tert-butoxycarbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid with triethylamine;N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate in dimethyl sulfoxide at 20; for 0.166667 h;
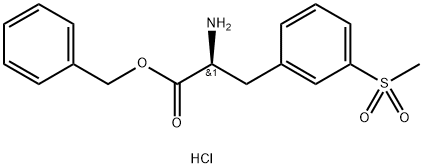
Stage #2:(2S)-2-amino-3-(3-(methanesulfonyl)phenyl)propionic acid benzyl ester hydrochloride in dimethyl sulfoxide at 20; for 18 h;
Stage #3: with hydrogenchloride in 1,4-dioxane at 20; for 0.5 h;
Steps:
A; B A) Preparation of compound IV-HCI
2-(tert-Butoxycarbonyl)-5,7-dichloro-1 ,2,3,4-tetrahydroisoquinoline-6-carboxylic acid (5 g, 14.4 mmol) was dissolved in a mixture DMSO (25 mL) and triethylamine (10.1 mL, 72.2 mmol). HATU (6.9 g, 18.1 mmol) was added. The mixture was stirred at room (0168) temperature for 10 min. Benzyl (S)-2-amino-3- (3-(methylsulfonyl)phenyl) propanoate hydrochloride (5.9 g, 15.9 mmol) was added and the mixture was stirred for 18 h at room temperature. Isopropyl acetate (50 mL) and water (50 mL) were added. The phases were separated and the organic phase was washed with water (50 mL) and then with 1 N HCI (50 mL). Dioxane (100 mL) was added and the isopropyl acetate was distilled. 4M HCI in dioxane (10 mL) was added and the mixture was stirred at room temperature for 30 min. The solid obtained was filtered, washed with dioxane, and dried in vacuo at 45 °C to give 8.69 g (99% yield) of the desired product (IV-HCI) as a white solid (HPLC purity: 97%). ; Compound IV-HCI as obtained in the previous step (20 g, 33 mmol) was dissolved in a mixture of DCM (200 mL) and triethylamine (4.7 mL). The DCM phase was washed with water (200 mL). Dioxane (400 mL) was added and the DCM was distilled off. Then, 4M HCI in dioxane (10 mL) was added and the mixture was stirred at room temperature for 1 .5 h. The solid obtained was filtered, washed with dioxane, and dried under vacuum at 45 °C to yield 19.5 g (98% yield) of the desired product (IV-HCI) as a white solid (HPLC purity: 99.4%).
References:
INTERQUIM, S.A.;BERZOSA RODRíGUEZ, Xavier;MARQUILLAS OLONDRIZ, Francisco WO2019/20580, 2019, A1 Location in patent:Page/Page column 22; 23
1194550-63-4
4 suppliers
inquiry
![L-Phenylalanine, N-[(5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester, hydrochloride (1:1)](/CAS/20211123/GIF/1194550-65-6.gif)
1194550-65-6
55 suppliers
inquiry
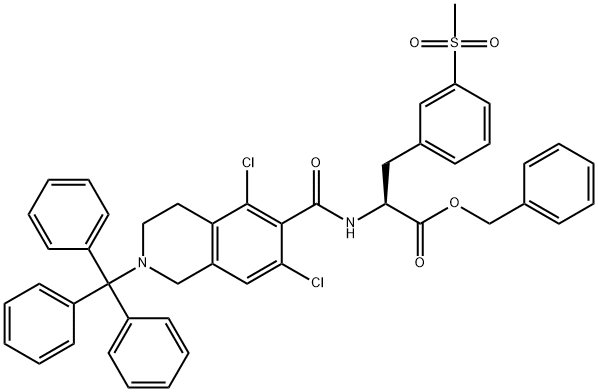
![2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-[[[(1S)-1-[[3-(methylsulfonyl)phenyl]methyl]-2-oxo-2-(phenylmethoxy)ethyl]amino]carbonyl]-, 1,1-dimethylethyl ester](/CAS/20180703/GIF/1194550-61-2.gif)
1194550-61-2
28 suppliers
inquiry
![L-Phenylalanine, N-[(5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester, hydrochloride (1:1)](/CAS/20211123/GIF/1194550-65-6.gif)
1194550-65-6
55 suppliers
inquiry
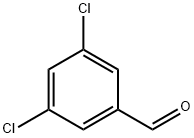
10203-08-4
294 suppliers
$8.00/5g
![L-Phenylalanine, N-[(5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester, hydrochloride (1:1)](/CAS/20211123/GIF/1194550-65-6.gif)
1194550-65-6
55 suppliers
inquiry
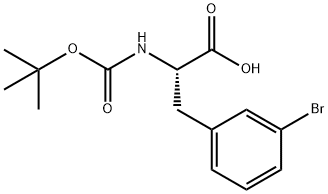
82278-73-7
181 suppliers
$40.00/1G
![L-Phenylalanine, N-[(5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester, hydrochloride (1:1)](/CAS/20211123/GIF/1194550-65-6.gif)
1194550-65-6
55 suppliers
inquiry